Hepatitis C virus was identified in 1989 as a major cause of the parenterally transmitted non-A non-B hepatitis. Chronic hepatitis C virus infection affects an estimated 170 million people worldwide1 and is characterised by varying degrees of inflammation and hepatic fibrosis. A proportion of patients with chronic infection will develop progressive liver damage with cirrhosis and complications of end stage liver disease over 20 to 40 years. Chronic infection is now the leading indication for liver transplantation in developed nations and will continue to pose an important health and economic burden during the next 10 to 20 years. Here we outline the criteria for screening, diagnosing, and treating patients with hepatitis C virus infection and describe potential future therapies.
Prevalence and transmission of hepatitis C virus infection
Hepatitis C virus (see bmj.com for description of virus) is transmitted by parenteral or permuscosal exposure to infected blood or body fluids. Many patients will give a history of injecting drug use or transfusion of blood products before the implementation of antihepatitis C virus screening of blood donors in 1992. Seroprevalence among injecting drug users is more than 80%, and this remains a major risk factor for newly acquired hepatitis C virus infection in the developed world. Community based strategies for prevention of infection in these high risk groups are needed urgently but depend on resources (box 1). Screening of volunteer blood donations in developed nations has significantly reduced transfusion related hepatitis. Most countries in the developing world do not, however, have adequate screening procedures, and only about 40% of donated blood is tested for the virus. Occupational, vertical, and sexual transmission account for only a minority of new cases of hepatitis C virus infection. Sexual transmission of hepatitis C virus among monogamous partners is rare, but testing is often carried out for reassurance (box 2). See bmj.com for contact details for information on screening recommendations.
Box 1 Prevention strategies to reduce transmission of hepatitis C virus through injecting drug use
Increased education of healthcare providers in prevention and treatment of substance misuse
Access to sterile syringes and needle exchange programmes
Community based programmes for injecting drug users
Education in safe injecting practice
Coordinated medical, psychiatric, and social services
Access to counselling and healthcare services for prisoners
Summary points
Complications of liver disease related to hepatitis C virus infection are expected to increase over the next 10 to 20 years
Prevention strategies need to be implemented to reduce person to person spread in high risk groups
Sustained viral eradication and prevention of disease progression is possible through antiviral therapy
Current optimal treatment is pegylated interferon alfa and ribavirin for 24 or 48 weeks on the basis of genotype and virological response
Newer specific targeted antiviral therapies for hepatitis C infection are in clinical development
Clinical course and disease progression
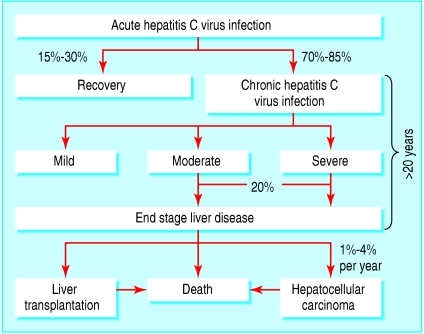
Acute hepatitis C virus infection is usually subclinical, and there are no reliable predictive factors for chronic infection. The relatively small size of the virus's RNA polyprotein, rapid viral replication, and high mutation rates all contribute to the virus's genetic heterogeneity and allow it to escape the host's immune response, resulting in chronic infection for most patients (figure). Progression of the disease is variable, and despite inherent limitations, histological evaluation of serial liver biopsy specimens remains the only reliable method to determine changes in severity of disease over time.2 On the basis of various study designs and models that predict rates of fibrosis progression, 20% to 30% of patients may be expected to develop cirrhosis over 20-30 years. This is reflected in the steady rise in the incidence of complications related to chronic liver disease, such as hepatocellular carcinoma, in many countries that are beginning to reach peak hepatitis C virus seroprevalence, such as Japan. Several host and viral factors affect disease progression, although determinants of individual risk and precise mechanisms of liver injury have yet to be determined (box 3). Better understanding of host-viral interactions may allow for targeted antiviral or other therapy aimed primarily at those at greatest risk of disease progression.
Figure 1.
Hepatitis C virus persists in most patients with acute hepatitis C virus infection, and some develop progressive hepatic injury and subsequent complications of end stage liver disease
Box 2 Screening recommendations for hepatitis C virus infection2
History of injecting drug use
Conditions with high seroprevalence of hepatitis C virus infection
HIV infection
Patients receiving haemodialysis for end stage renal disease
Haemophilia
Unexplained elevated aminotransferase levels or chronic liver disease
Transfusion of blood products or organ transplantation before 1992
Children born to mothers infected with hepatitis C virus
Healthcare workers with needlestick injury or exposure of mucous membranes to hepatitis C virus positive blood
Current sexual partners of people infected with hepatitis C virus
Diagnosis of hepatitis C virus infection
Diagnostic tests for hepatitis C virus infection include serological assays for antibodies and molecular techniques for detecting virus particles. Automated enzyme immunoassays allow for processing of large numbers of samples and are used mainly for screening of blood and initial detection of antihepatitis C virus antibodies. In clinical practice, false negative antibody test results may occur in immunocompromised patients or those with renal failure or hepatitis C virus associated cryoglobulinaemia. Positive antibody test results require confirmation of hepatitis C virus RNA using a molecular amplification assay. Several quantitative and qualitative assays are available with dynamic ranges between 101 and 107 IU/ml. Quantitation of hepatitis C virus RNA provides important prognostic information on the likelihood of response to antiviral therapy. A negative test result for hepatitis C virus RNA in the presence of a positive antibody test result is seen occasionally in practice and indicates either a resolved infection; a false positive immunoassay, which can occur in low risk populations; or, rarely, an intermittent low level viraemia.
Box 3 Potential factors influencing progression of fibrosis
Viral factors
Hepatitis C virus RNA load*
Genotype 1b*
Quasispecies diversity*
Hepatitis C virus proteins
Host factors
Age at infection
Sex
Race*
Coinfection (hepatitis B virus, HIV-1)
Comorbid disease (iron overload, non-alcoholic steatohepatitis, schistosomiasis)
Genetic polymorphisms
Disease expression (elevated levels of alanine aminotransferase, stage of fibrosis at diagnosis)
Metabolic factors (obesity, insulin resistance, steatosis)
Immunosuppression (organ transplantation)
Other factors
Alcohol
Smoking*
Environmental toxins
*Possible association with disease progression only (based on inconclusive studies)
Despite inherent limits of liver biopsy, assessing necroinflammatory activity and liver fibrosis provides useful information for determining requirements for antiviral therapy, prognosis, and the potential for progression of fibrosis, and for evaluating comorbid states such as steatosis, drug induced liver injury, or iron overload. Whether patients with better prognostic indicators, such as those infected with genotypes 2 and 3 or with persistently normal transminase levels, require a liver biopsy for treatment decisions is debatable. Most experienced clinicians are likely to recommend liver biopsy unless there are obvious contraindications. Secondary end points of therapy, such as fibrosis regression, are likely to assume greater importance in the future given the ever increasing proportion of patients that have failed to achieve sustained virological responses to currently available therapy. Serodiagnostic and other surrogate markers of fibrosis are in development, and their clinical utility in replacing the qualitative information provided by a biopsy for hepatitis C virus infection remains to be determined.3,4 They are, however, in widespread clinical use in several countries.
Treatment decisions
The main aim of treating patients with chronic hepatitis C virus infection is to prevent progressive hepatic fibrosis by eradicating viral RNA. Sustained virological clearance is defined as the absence of hepatitis C virus RNA as judged by a sensitive polymerase chain reaction assay 24 weeks after the end of treatment. The potential long term benefits of sustained virological clearance include normalisation of serum aminotransferase levels, improvement in hepatic necroinflammation and fibrosis, improvement in health related quality of life measures, survival benefits, and reduction in risk of developing hepatocellular carcinoma. Although all patients should be considered for treatment, balancing the risks of progressive disease with potential side effects of current therapy remains a major challenge for healthcare providers (box 4). Thus patients with mild disease activity should be given the option of deferring therapy.
Several factors are predictive of favourable virological responses to standard antiviral therapy, a combination of pegylated interferon and ribavirin (see box A on bmj.com). Viral genotype and baseline hepatitis C virus RNA levels seem to be the most important predictors of response. More than 80% of patients infected with genotype 2 or 3 can be expected to eradicate the virus after six months of therapy. Unfortunately, genotype 1 infection predominates in developed countries, optimal treatment is often not suitable or available to all patients, and response rates in clinical practice are much lower than those predicted from multicentre clinical trials using patient cohorts who have been carefully selected and are motivated.
Treatment options
The current standard of care for treating previously untreated patients with chronic hepatitis C virus infection is combination pegylated interferon alfa by subcutaneous injection once weekly and oral ribavirin daily. The interferons are a group of naturally occurring cytokines that exhibit a variety of immunomodulatory, antiproliferative, and antiviral effects. Ribavirin is a purine nucleoside that has antiviral effects against hepatitis C virus only when combined with interferon alfa. Pegylation refers to the covalent attachment of an inert, water soluble polymer of polyethylene glycol to the interferon molecule, allowing for improved pharmacokinetic profiles and once weekly dosing. Two pegylated interferon alfa preparations are available: pegylated interferon alfa-2b, administered at a weight based, 1.5 μg/kg dose, and pegylated interferon alfa-2a, administered at a fixed, 180 μg dose. Combination pegylated interferon alfa and ribavirin therapy can achieve a sustained virological response in 54%-56% of patients, including 42%-46% of patients with genotype 1 infection and about 80% of those with genotype 2 or 3 infection.
The results of clinical trials support a recommendation that patients with genotype 1 require 48 weeks of pegylated interferon therapy with daily ribavirin (1000-1200 mg dosed according to weight). Patients with the more treatment favourable genotypes 2 and 3 need be treated for only 24 weeks with 800 mg of ribavirin daily.5-7 Given that many of the large clinical trials have been based in Europe or North America, data on treatment efficacy for genotypes 4-6 are limited. As a result, these patients are treated as for genotype 1 infection (see fig C on bmj.com). A recent technical review from the American Gastroenterological Association provides further details on management options in patients infected with hepatitis C virus.8
Box 4 Recommendations for interferon based therapy
Accepted for therapy
Age > 18 years
Persistently abnormal alanine aminotransferase levels
Compensated liver disease (total serum bilirubin <25 μmol/l; prothrombin time international normalised ratio < 1.5; albumin > 34 g/l; platelet count > 75 000 × 10/l; no hepatic encephalopathy, bleeding gastroesophageal varices, or ascites)
Liver biopsy consistent with chronic hepatitis and at least portal fibrosis with moderate inflammation
Previous suboptimal treatment for chronic hepatitis C virus infection
Individualised treatment
Normal alanine aminotransferase levels
Non-responders to optimal therapy
Substance misuse
Mild or no fibrosis with minimal activity on liver biopsy
Coinfection with HIV-1 or hepatitis B virus
Acute hepatitis C virus infection
Age < 18 years
Post liver transplantation
Deferred or contraindicated
Poorly controlled major depressive or important psychiatric illness
Comorbid states exacerbated by inteferon (autoimmune hepatitis, untreated hypothyroidism)
Renal, heart, or other solid organ transplant (low dose pegylated interferon before renal transplantation; interferon therapy contraindicated after renal transplantation*)
Pregnant or unable to comply with barrier contraception Significant comorbidity (for example, severe cardiac or pulmonary disease)
Decompensated liver disease
Haemoglobin disorders
Active or suspected malignancy
Chronic renal insufficiency
*No data on standard or pegylated interferon in other solid organ transplants. Newer therapies should be awaited
Assessing early response
Monitoring of hepatitis C virus RNA levels during treatment should be carried out with the same quantitative amplification assay as used before treatment. Failure to achieve an early virological response (≥ 2 log10 decline or loss of viral RNA at week 12 of treatment) in patients infected with genotype 1 is a good predictor of non-response to continued therapy. These early stopping rules reduce costs, may provide an incentive for patients to adhere to the prescribed treatment regimen, and help avoid prolonged exposure to drugs that are likely to have minimal benefits on virological response. Likewise, 12 weeks of therapy may also suffice in patients with genotypes 2 or 3 with undetectable viral RNA levels at week 4.
Additional educational resources
American Association for the Study of Liver Diseases (www.aasld.org/eweb/docs/hepatitisc.pdf)—practice guidelines on diagnosis, management, and treatment of chronic hepatitis C virus infection
British Society of Gastroenterology (www.bsg.org.uk/clinical_prac/guidelines/hep_c.htm)—clinical guidelines for management of chronic hepatitis C virus infection, with subsequent addendum to incorporate pegylated interferon therapy (Dienstag and McHutchison 2006 provides more recent treatment guidelines from the American Gastroenterology Association compared with the British Society of Gastroenterology from 2001 and 2003)
Veterans Affairs national hepatitis C programme (www.hepatitis.va.gov/)—useful educational resources including clinician tools, treatment guidelines, and handouts for patients
Information for patients
Centers for Disease Control (www.cdc.gov/idu/hepatitis/index.htm)—provides fact sheets on viral hepatitis and injecting drug users (other educational materials are available at www.cdc.gov/ncidod/diseases/hepatitis/c/index.htm)
American Academy of Family Physicians (http://familydoctor.org/handouts/071.html)—detailed answers to patients' frequently asked questions regarding hepatitis C virus infection
National Institutes of Diabetes and Digestive and Kidney Diseases (http://digestive.niddk.nih.gov/ddiseases/pubs/hepc_ez/)—patient resource with diagrams and details providing a general overview of hepatitis C virus infection (more detailed information on management is available at http://digestive.niddk.nih.gov/ddiseases/pubs/chronichepc/)
Individualising treatment
Given the influence of various host and viral characteristics on virological responses to interferon based therapy, treatment schedules often need to be individualised in clinical practice. For example, a patient with hepatitis C virus genotype 2 infection may have a higher sustained virological response rate than a patient with genotype 3 infection and high levels of hepatitis C virus RNA or advanced fibrosis. Thus clinicians may wish to consider longer duration of therapy on an individual basis, taking into account such considerations as high viral burden, advanced disease stage, or delayed response to therapy. Patients with mild disease and persistently normal transaminase levels seem to be at low risk of disease progression, and one option in these patients is watchful waiting along with periodic monitoring of liver tests. Such decisions to defer treatment should be based not only on biopsy results but also patient preferences and motivation.
Chronic hepatitis infection: first steps
Patients with chronic hepatitis C virus infection are often asymptomatic; positive antibody test results for hepatitis C virus in high risk groups require confirmation with a hepatitis C virus RNA assay
All patients should be considered as potential candidates for antiviral therapy and managed in appropriate multidisciplinary settings
Adherence to the initial treatment course provides the best opportunity for sustained eradication of the virus
Consider referral of non-responders to tertiary centres for participation in clinical trials of newer therapies
Management of side effects of antiviral therapy
Side effects to current antiviral therapy are relatively common and range from mild, non-specific, flu-like symptoms to major neuropsychiatric disturbance. Early recognition and management of symptoms is vital in maintaining adherence to therapy. This requires frequent follow-up and a good relationship between healthcare provider and patient. A multidisciplinary approach involving allied healthcare professionals and support groups should be adopted, along with adjunctive use of simple antipyretics, growth factors, and antidepressants to ensure compliance to the prescribed regimen.9 Such strategies to improve adherence may result in better virological response rates in many patients (see box B on bmj.com).10
Other patient populations
Our current understanding of outcomes to antiviral therapy is essentially based on data from clinical registration trials that include highly selected patient groups. The clinical course and pathogenesis of hepatitis C virus infection can vary in different populations. For example, patients with persistently normal transaminase levels and mild disease at baseline tend to have slower disease progression, and many physicians, in consultation with their patients, opt for close monitoring without therapy. Patients with advanced disease are clearly appropriate candidates for therapy, but they must have stable, preserved hepatic function. The role of long term maintenance pegylated interferon in reducing clinical complications of end stage liver disease is being evaluated in non-responders to therapy, and preliminary results seem to indicate some benefit. Non-responders to prior standard interferon and ribavirin therapy can be retreated with pegylated interferon and ribavirin with an incremental gain in sustained virological response of about 10%, but retreatment requires consideration of disease stage and patient tolerance to prior treatment. For reasons that are not entirely clear, certain patient populations, such as African Americans or those coinfected with HIV, seem to have lower sustained virological response rates. Other patient groups may not be suitable for optimal combination therapy, including patients with end stage renal disease or haemoglobinopathies. An increasing number of patients have failed to achieve sustained responses to current standard of care with pegylated interferon and ribavirin. Therapeutic options are limited for these patients and involve adopting lifestyle measures to reduce the effect of risk factors such as alcohol and steatosis on disease progression. Longer term pegylated interferon or participation in trials of novel therapies are possibilities but may not be viable options for many patients (see box C on bmj.com).11 Useful educational materials and other patient related information can be obtained through various government and non-profit agencies (see bmj.com).
Future treatment options
The lack of effective cell culture systems and small animal models has limited progress in our understanding of the host-viral interaction in hepatitis C virus infection. The recent development of genetic constructs and infectious clones capable of replicating in cell lines, along with studies in related viruses, has led to the identification of several novel therapeutic targets and the subsequent development of hepatitis C virus specific antiviral compounds. Therapeutic options that are promising and currently in clinical trials include newer interferons, alternatives to ribavirin, immune modulators, and specific hepatitis C virus enzyme inhibitors. Although resistance and potential toxicity issues with some of the newer enzyme inhibitors still require further resolution and understanding, therapeutic options in the future will involve simultaneously disrupting several pathways in the hepatitis C virus lifecycle, followed by modulation of the host immune system to clear virus and maintain long term response.12
Conclusions
Hepatitis C virus infection is a global health problem that leads to significant morbidity and mortality from complications of end stage liver disease., Public education, implementation of primary prevention methods, healthcare access, and identification of hepatitis C virus infected patients at greatest risk of disease progression remain important challenges to health policymakers and practitioners alike. The recent development of novel and specific targeted antiviral therapies for hepatitis C infection provides some cause for renewed optimism in the future for patients that have failed to achieve adequate responses or remain ineligible for current treatment regimens.
Supplementary Material
 Additional details are on bmj.com
Additional details are on bmj.com
We thank Jennifer King of August Editorial for help in preparing this manuscript. Basic information on available serological tests for hepatitis C virus is available through the National Institutes of Health (http://digestive.niddk.nih.gov/ddiseases/pubs/chronichepc).
Contributors: All authors contributed equally to the writing of this clinical review. KP is guarantor.
Funding: None.
Competing interests: AJM has received a grant and research support from Schering-Plough and Hoffman-La Roche. JMcH has received a grant and research support from Schering-Plough and Hoffman-La Roche and advisory and consulting fees from Schering-Plough.
References
- 1.World Health Organization. Hepatitis C. Factsheet No 164 (updated Oct 2000). www.who.int/mediacentre/factsheets (accessed 3 Feb 2006).
- 2.Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology 2002;36(suppl 1): S152-60. [DOI] [PubMed] [Google Scholar]
- 3.Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol 2004;99: 1160-74. [DOI] [PubMed] [Google Scholar]
- 4.Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology 2006;43(suppl 1): S113-20. [DOI] [PubMed] [Google Scholar]
- 5.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358: 958-65. [DOI] [PubMed] [Google Scholar]
- 6.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347: 975-82. [DOI] [PubMed] [Google Scholar]
- 7.Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004;140: 346-55. [DOI] [PubMed] [Google Scholar]
- 8.Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology 2006;130: 231-64; quiz 214-7. [DOI] [PubMed] [Google Scholar]
- 9.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA 2002;288: 2868-79. [DOI] [PubMed] [Google Scholar]
- 10.Sethi A, Shiffman ML. Approach to the management of patients with chronic hepatitis C who failed to achieve sustained virologic response. Clin Liver Dis 2005;9: 453-71, vii-viii. [DOI] [PubMed] [Google Scholar]
- 11.McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology 2002;123: 1061-9. [DOI] [PubMed] [Google Scholar]
- 12.McHutchison JG, Bartenschlager R, Patel K, Pawlotsky JM. The face of future hepatitis C antiviral drug development: recent biological and virologic advances and their translation to drug development and clinical practice. J Hepatol 2006;44: 411-21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.