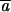Abstract
Spontaneous mutations play a fundamental role in the maintenance of genetic variation in natural populations, the nature of inbreeding depression, the evolution of sexual reproduction, and the conservation of endangered species. Using long-term mutation-accumulation lines of the nematode Caenorhabditis elegans, we estimate the rate and magnitude of mutational effects for a suite of behaviors characterizing individual chemosensory responses to a repellant stimulus. In accordance with evidence that the vast majority of mutations are deleterious, we find that behavioral responses degrade over time as a result of spontaneous mutation accumulation. The rate of mutation for behavioral traits is roughly of the same order or slightly smaller than those previously estimated for reproductive traits and the average size of the mutational effects is also comparable. These results have important implications for the maintenance of genetic variation for behavior in natural populations as well as for expectations for behavioral change within endangered species and captive populations.
AS the ultimate source of novel variation upon which selection can act to produce adaptation to temporally and spatially variable environments, mutation is essential to the evolution and persistence of natural populations (Lynch and Walsh 1998). Spontaneous mutations are thought to play a fundamental role in maintenance of genetic variation in natural populations, the nature of inbreeding depression, and the evolution of sexual reproduction (Lynch et al. 1999). The accumulation of slightly deleterious mutations has also been shown to pose a potentially serious risk of extinction in small, isolated natural populations due to the increased efficacy of genetic drift (Lynch et al. 1999). Captive populations may experience a similar threat as a result of being maintained for prolonged periods under benign conditions where selection against newly arising mutants is relaxed (Bryant and Reed 1989). Thus, a better understanding of the nature of spontaneous mutation accumulation (MA), in addition to furthering our understanding of a variety of evolutionary processes, will be an essential component in assessing the consequences of mutation for the genetic integrity of endangered populations and of our own. As of yet, however, the database on the characteristics of spontaneous mutation in natural populations remains limited in the number of both taxa and traits represented. Most of the studies conducted to date have focused on life-history characters such as survival and reproduction, finding that genomic mutation rates are on the order of 0.01–1.0/individual/generation in most higher organisms and that the majority of mutations arising have negative effects on fitness (Lynch et al. 1999; although see Shaw et al. 2002; Keightley and Lynch 2003). Despite the broad implications of spontaneous deleterious mutation and the large number of genes underlying behavior that have been revealed by direct mutagenic approaches in a variety of organisms, the rate of spontaneous mutation for behavioral traits has never been estimated.
The goal of this study was to obtain estimates of the basic mutation parameters for a suite of behavioral traits expected to have direct impact on individual fitness. Chemotaxis in the free-living soil nematode Caenorhabditis elegans provides such a system. Chemosensation is the major means through which nematodes interact with the environment, with 5–10% of the C. elegans genome devoted to chemosensory function (Bargmann 1998). For example, chemotaxis is known to play a vital role in the avoidance of predators, parasites, and toxins; the location of food, hosts, and mates; several steps in the male-mating pathway; and population regulation in response to increased density and resource depletion (Troemel 1999). Analysis of the molecular evolution of key components of the chemosensory pathway also suggests that chemotaxis is under strong selection (Jovelin et al. 2003; Jovelin and Phillips 2005).
C. elegans chemotaxes within a chemical gradient by performing a series of pirouettes (turns), which orient the worm toward the gradient, interspersed with a long series of sinusoidal swimming motions, whereby the worm navigates along the gradient in the direction to which it has just oriented (Pierce-Shimomura et al. 1999, 2001). This mode of chemotaxis is very similar to that used by bacteria (Falke et al. 1997). We have quantified these different characteristics of chemotaxis behavior using a computerized tracking system (Pierce-Shimomura et al. 1999) (Figure 1) in which we scored “directness” (the beeline distance divided by the total path length), average instantaneous velocity, and average turn rate in the presence of the repellant linoleic acid. We chose linoleic acid, a fatty acid with nematicidal properties that has been isolated from live cultures of Bascidiomycetes (Stadler et al. 1994), because of the likelihood that it is, or resembles, a compound that nematodes encounter and respond to in their natural environment.
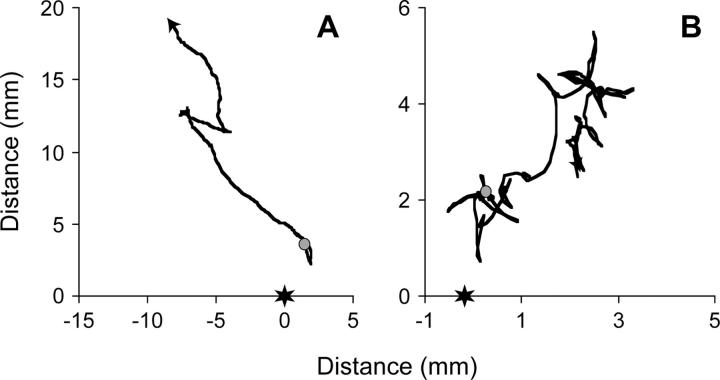
Figure 1.—
Computerized tracking of nematode behavior in response to a repellent chemical (linoleic acid, located at the star). Lines show the path taken by an individual nematode over a 4-min period, beginning at the circle and ending at the arrowhead. (A) An individual from the ancestral control line showing a clear avoidance response. (B) An individual from one of the mutation accumulation lines that showed low avoidance scores. Directness is given by the beeline distance divided by the total path length. Velocity and turn rate are estimated, respectively, using instantaneous changes in speed and angle as the individual navigates away from the chemical. Note the change in scale between the two graphs.
This study extends our previous work (Vassilieva et al. 2000) on mutation accumulation by focusing on a new class of traits, behavior. In doing so, we emphasize the need to account for ecologically relevant traits, in addition to life history, that may have large direct and indirect effects on fitness through their influence on resource acquisition, habitat selection, and mate choice.
MATERIALS AND METHODS
Mutation accumulation:
The MA experiment was initiated from a single highly inbred individual of the N2-Bristol wild-type strain (Vassilieva et al. 2000). Offspring of that individual were used to establish 100 replicate lines that were subsequently propagated by the transfer of a single, randomly selected individual across generations. The practice of repeated bottlenecking was employed to minimize the efficiency of natural selection against newly arising mutations. Concurrently with line division, several thousand individuals were frozen at −80° for use as an ancestral control to be assayed in parallel with the experimental lines. Between assays, worms were cultured at 20° on petri plates containing NGM agar seeded with an ∼80- to 90-μl suspension of OP50 Escherichia coli (Brenner 1974). At the time of the behavioral assay the lines had undergone an average of 370 generations of mutation accumulation. Extinction over this time period resulted in 67 extant lines that were used for the behavioral analysis.
Behavioral analysis:
To initiate the behavioral assay, a single base control line was established from frozen stock and propagated for four generations by single-individual bottlenecks to prevent maternal effects of freezing and to ensure homozygosity. That line was maintained via single-individual transfers for the duration of the experiment and used to establish the 67 control pseudolines assayed in parallel with the mutation accumulation lines. Chemotactic behavior was then quantified by taking three distinct measurements of individual responses to the repellant for five randomly selected progeny descended from the founding individual of each MA and N2 line. Directness was defined so as to be independent of both time and absolute distance traveled to control for the likelihood that some of the experimental lines had acquired mutations affecting their ability to move effectively but not necessarily to detect and orient to odorants. It serves as a measure of how efficiently the chemotaxis system functions in moving the individual away from a noxious source. A turn was defined as a change in direction of 90° or more. Previous studies have shown that turn rate increases when worms are oriented opposite a gradient (Pierce-Shimomura et al. 1999, 2001). Thus we expected turn rate to be increased in mutant, relative to control, individuals. Velocity, the average speed attained by the worm over the course of the assay, was our indicator of general locomotory ability.
Each assay commenced with the transfer of a single L4 individual to the center (origin) of a clean 10-cm petri dish containing chemotaxis agar (Bargmann et al. 1993) and the addition of 1 μl of a 1% linoleic acid (Sigma, St. Louis) solution (freshly diluted in ethanol) adjacent to the worm. The position of the worm was recorded at 1-sec intervals using a compound microscope equipped with a video camera and a motorized stage controlled by a computer running Image Pro Plus (Media Cybernetics) image analysis software (Pierce-Shimomura et al. 1999). MA and N2 individuals were assayed in an alternating fashion to correct for any fluctuations in environmental conditions over the course of the day. Individuals from different lines were interspersed during the assays so that any temporal effects, including recovery from larval lethargus, would be randomized across lines. A series of assays was also conducted in the absence of the repellant to assess the baseline locomotory response without an olfactory cue.
Statistical analysis:
A slight temporal trend across days in the turn rate and velocity data was corrected by regressing the control line scores on day and then using the residuals for subsequent analysis. Mutational heritability was estimated as the ratio of the among-mutant line variance over the average within-line variance, divided by twice the number of mutation-accumulation generations (Lynch and Walsh 1998) (2 × 370 in this case). Mutation-rate parameters were estimated both by comparing the change in mean of the mutant lines to the change in the among-line variance [the Bateman-Mukai (B-M) approach; Lynch and Walsh 1998] and by using a maximum-likelihood approach based on sampling from the actual distribution of the control lines (outlined in detail in Vassilieva et al. 2000). The B-M approach is expected to yield downwardly biased estimates of mutation rate and upwardly biased estimates of mutation effect size (Lynch and Walsh 1998; García-Dorado and Gallego 2003; Keightley 2004). Models assuming both mutations with constant effect (MLc) and mutations with variable effects drawn from a gamma distribution (MLv) were fit. The improvement of the fit of the latter over the former was tested using a χ2 test of twice the difference of the likelihoods with 1 d.f.. Confidence intervals on the mutational heritabilities and B-M mutation estimates were obtained by bootstrapping over the MA lines, while those for the maximum-likelihood estimates were determined by a two-unit decrease from the maximum on the likelihood surface. Directness was arcsine square root transformed and velocity and turn rate were square root transformed before estimation of mutational parameters to greatly enhance the normality of the error distribution and to improve performance in a subsequent analysis of mutation correlation structure (see Estes et al. 2005, accompanying article in this issue). Estimates based on the untransformed scale are not significantly different.
RESULTS
After 370 generations of mutation accumulation, all three behavioral measures declined significantly in the mutant lines as compared to the ancestral population (which was assayed contemporaneously after having been frozen during the intervening period of time). Individuals accumulating spontaneous mutations were less direct in their avoidance paths, moved more slowly, and turned more frequently than the nonmutant controls (Table 1, Figure 2). For directness and turn rate, the mutant lines exhibited behavioral phenotypes approaching those of the control line in the absence of the repellant (Figure 2). Further, mutant lines were slower than the ancestral control line under all conditions. This overall reduction in chemotaxic and locomotory ability was accompanied by a significant increase in the variation among the different mutant lines, as expected (Figure 3; Table 1).
TABLE 1.
Means and rates of change in means for characters in control and MA lines and mutational heritabilities
| Character | MeanControl | MeanMA | Rm | % change | h2m |
|---|---|---|---|---|---|
| Directness | 0.264 (0.007) | 0.213 (0.007) | −0.0001 (2.32 × 10−5) | −0.05 | 0.0002 (0.0001) |
| Velocity | 8.41 (0.17) | 7.84 (0.19) | −0.0015 (0.0007) | −0.02 | 0.0004 (0.0002) |
| Turn rate | 4.66 (0.16) | 6.70 (0.25) | +0.0055 (0.0012) | +0.12 | 0.0012 (0.0007) |
| Early productivity | 44.01 (1.701) | 19.28 (0.701) | −0.0666 (0.0051) | −0.15 | 0.0017 (0.0004) |
| Late productivity | 163.5 (4.191) | 105.1 (2.326) | −0.1607 (0.0168) | −0.10 | 0.0017 (0.0004) |
| r | 1.507 (0.012) | 1.095 (0.018) | −0.0011 (0.0001) | −0.74 | 0.0011 (0.0003) |
| Width | 0.056 (0.0004) | 0.051 (0.0003) | −1.311 × 10−5 (2.02 × 10−6) | −0.23 | 0.0011 (0.0003) |
Means for each character in the control and MA lines per generation rates of decline in mean phenotype (Rm) observed for the MA lines, Rm standardized by the control mean (% change), and mutational heritabilities (h2m), with one standard error in parentheses. Estimates for the life-history and body size traits are provided for comparison and are described in Estes et al. (2005)(accompanying article).
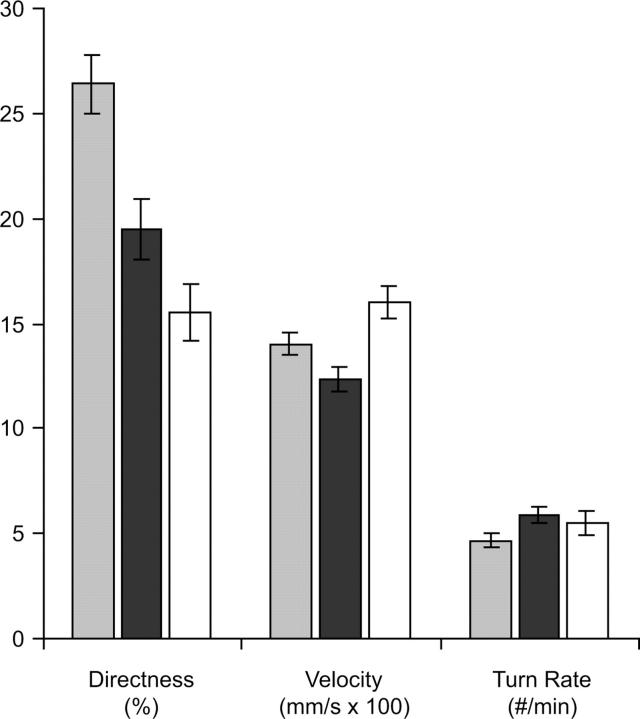
Figure 2.—
Change in behavior after 370 generations of mutation accumulation. Average values for the unmutated control (shaded bar), the mutation accumulation lines (solid bar), and the control in the absence of the repellent chemical (open bar) are given. Behavior strongly declines under the accumulation of spontaneous mutations, either becoming more similar to the control in the absence of a stimulus (directness and turn rate) or simply showing an overall degradation in response (velocity). Error bars are 95% C.I.
Figure 3.—
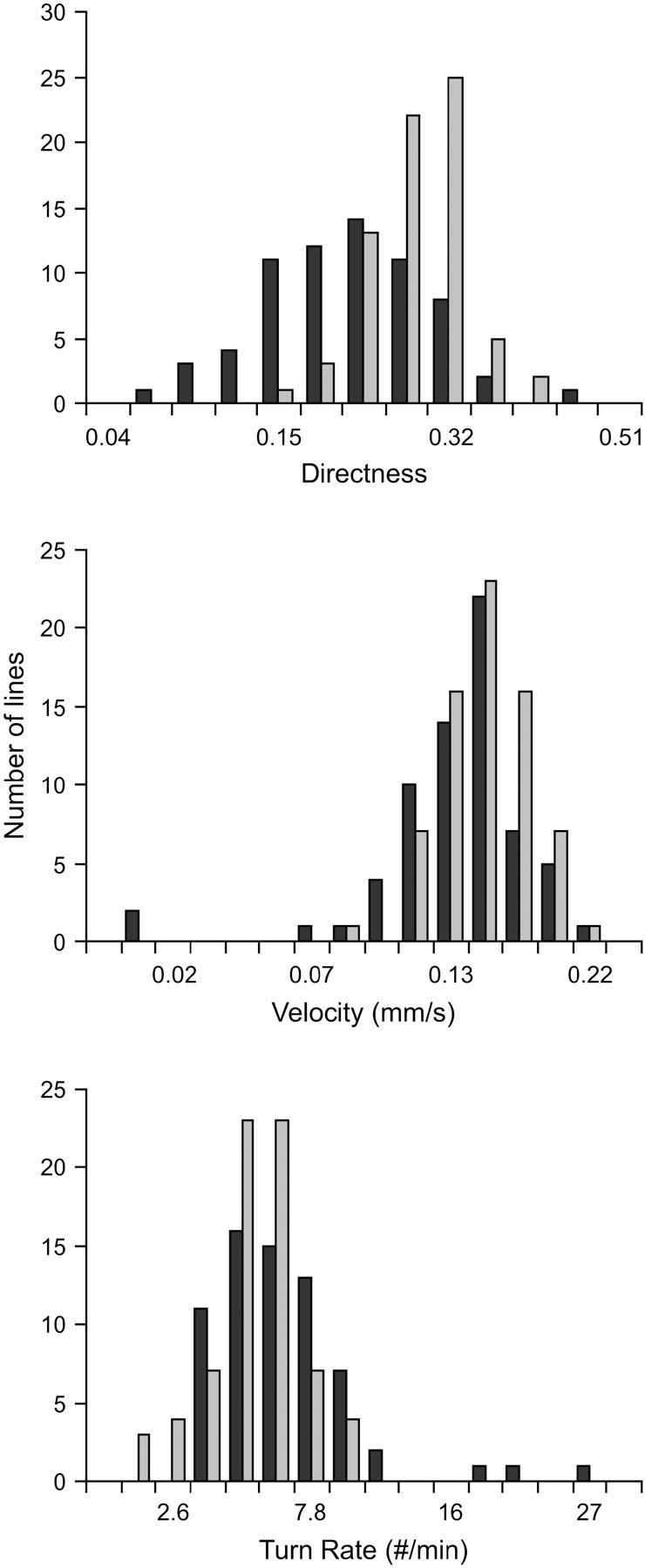
Change in variance among lines under mutation accumulation. Pseudoreplicate control lines (shaded bars) provide estimates of the among-line environmental variance. The increase in variance beyond that shown by the mutation accumulation lines (solid bars) provides estimates of the genomic mutation rate and the average size of mutational effects.
The pattern of decline in mean phenotype and increase in among-line variance is qualitatively very similar to results obtained for morphological and life-history characters in a wide variety of organisms (Houle et al. 1996; Lynch et al. 1999). The rates of change in mean phenotype (∼0.1%/generation) and mutational heritabilities for these traits are on the same order or slightly smaller than those reported for life-history and morphological characters within these same experimental lines (Table 1; Vassilieva et al. 2000; Azevedo et al. 2002; Estes et al. 2005, accompanying article). Estimates for the total genomic mutation rate were also on the same order or somewhat smaller (U = 10−2 − 10−3; Table 1), as were the average homozygous effect sizes of each mutation (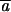 = 8–40%; Table 2). Although the variable effects model fits better for velocity and turn rate (thereby yielding larger estimates for mutation rate), estimates derived using different approaches are not significantly different from one another for any of the traits (Table 2). The mutation rates estimated for the simple characters (velocity and turn rate) were smaller than that for the composite trait (directness), while their mutation effect sizes were larger, suggesting that these traits are influenced by relatively fewer genes, each with larger individual effects. Overall, then, we have shown that behavior is subject to an intensity of mutational pressure similar to that affecting morphology and life-history characters.
= 8–40%; Table 2). Although the variable effects model fits better for velocity and turn rate (thereby yielding larger estimates for mutation rate), estimates derived using different approaches are not significantly different from one another for any of the traits (Table 2). The mutation rates estimated for the simple characters (velocity and turn rate) were smaller than that for the composite trait (directness), while their mutation effect sizes were larger, suggesting that these traits are influenced by relatively fewer genes, each with larger individual effects. Overall, then, we have shown that behavior is subject to an intensity of mutational pressure similar to that affecting morphology and life-history characters.
TABLE 2.
Estimates of genomic mutation parameters
| Genomic mutation rate (U)
|
Average mutational effect size
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trait | UB-M | UMLc | UMLv |
|
|
|
CV(a) | |||
| Directness | 0.0177 | 0.0124 | −0.04 | −0.08 | ||||||
| (0.0046, 0.0344) | (0.0062, 0.0252) | (−0.08, −0.01) | (−0.14, −0.04) | |||||||
| Velocity | 0.0011 | 0.0025 | 0.0092 | −0.16 | −0.16 | −0.07 | 2.4 | |||
| (0.0004, 0.0022) | (0.0012, 0.0042) | (0.0016, 0.0164) | (−0.26, −0.06) | (−0.26, −0.13) | (−0.31, −0.05) | (1.3, 2.5) | ||||
| Turn rate | 0.0020 | 0.0024 | 0.0038 | 0.20 | 0.35 | 0.25 | 1.17 | |||
| (0.0008, 0.0036) | (0.0013, 0.0043) | (0.0013, 0.0064) | (0.09, 0.31) | (0.26, 0.41) | (0.18, 0.63) | (0.61, 1.20) | ||||
Estimates and 95% confidence intervals. Estimators: Mlc, maximum-likelihood estimates assuming constant mutational effects; Mlv, maximum-likelihood estimates assuming variable mutational effects. The variable effects model did not fit significantly better than the constant effects model for directness, but did fit better for velocity and turn rate using a chi-square test, P < 0.01. Mutational effect size values are relative to the control mean. CV(a) is the coefficient of variation for the effects under the variable effects model (MLv).
DISCUSSION
For many organisms, the ability to elicit appropriate behavioral responses to external environmental stimuli, such as cues indicating the presence or absence of food, predators, and conspecifics, as well as to physiologically tolerate conditions such as temperature, moisture, and UV, is an essential component of individual survival. With this in mind, we analyzed two closely related behavioral traits, chemotaxis and locomotion, in highly divergent lines of C. elegans to determine the mutability of these fitness-related behavioral traits. Because locomotion and olfaction play an analogous role in many other organisms, and because the latter is accomplished by very similar molecular mechanisms (Prasad and Reed 1999), the results observed here are generalizable from both ecological and mechanistic perspectives. The results of this study strongly indicate that behavior, and its degradation, are crucial considerations in ascertaining the total fitness consequences of mutation accumulation for natural populations.
Mutational parameters:
Estimates of mutational parameters for behavioral, morphological, and life-history traits in these lines are all fairly comparable to one another (Table 1; Vassilieva et al. 2000; Azevedo et al. 2002; Estes et al. 2005, accompanying article). A similar rate of mutational decline was observed for male motility in Drosophila melanogaster mutation accumulation lines after 30 generations, although estimates of mutation parameters could not be obtained in that study (Shabalina et al. 1997; see Lynch et al. 1999 for a critical discussion of this approach). This decline in behavioral response is also consistent with the genome-wide influence of EMS mutagenesis on male mating speed and phototaxis in D. melanogaster (Keightley and Ohnishi 1998). In general, the mutation rates obtained here are likely to underestimate the actual genomic mutation rate both because our analysis assumes that the majority of new mutations are deleterious (Shaw et al. 2002) (although there is no evidence for beneficial mutations here; Figure 3) and, more importantly, because there may be a large class of mutations that have effects that are too small to be detected in our assays (Davies et al. 1999; Estes et al. 2004). Furthermore, although the genomic mutation rates for behavior reported here are lower on an absolute scale than those for reproductive traits in some other organisms, C. elegans itself appears to have a relatively lower overall genomic mutation rate (Keightley and Caballero 1997; Vassilieva et al. 2000).
As evidenced by the relatively lower mutation rate and higher average effect size observed for velocity and turn rate, it appears that these two simple traits are under the control of relatively fewer genes of larger effect than life-history characters. Conversely, directness showed a quantitatively very similar pattern to life-history characters under mutation accumulation in C. elegans and other organisms. In the field, particularly in long-lived species, behavioral traits are often used as surrogates for direct measurements of survival and reproduction due to the difficulties involved in obtaining such estimates. Frequently, various aspects of locomotion such as sprint or burst speed are used to this end. These results suggest, however, that complex behaviors such as directness may show patterns of mutational degradation more similar to other classes of fitness-related traits and thus may be better suited than other more simple behavioral measures to answering some questions regarding fitness and fitness decline in natural populations in the absence of life-history data.
Implications for population viability:
Quantitative differences among traits not withstanding, our results indicate that behavioral traits are subject to significant mutational pressure on the same order as that for life-history and morphological characters. This strongly suggests that behavioral degradation is a potentially comparable source of fitness loss under conditions of mutation accumulation—one that has been largely absent in previous estimates of the deleterious consequences of recurrent mutation for natural populations. In endangered and captive-bred populations, then, the influence of behavioral degradation under mutation accumulation should be considered alongside other mutational effects when evaluating the impact that mutation might have on the long-term viability of these populations (Lande 1995; Lynch and O'Hely 2001).
Conclusions:
Although it remains to be seen whether the responses seen here for locomotion and olfaction can be regarded as typical for other behavioral traits (Shabalina et al. 1997), such as those influenced by learning, this work demonstrates that mutation accumulation can generate significant levels of individual variation in ecologically relevant behavioral traits within populations (Jovelin et al. 2003). The implications of this variation for individual populations will depend in large part on their specific genetic architecture and demographic characteristics, small and/or isolated populations being most at risk of experiencing behavioral degradation. Understanding the properties of newly arising mutations, then, will be crucial to elucidating the genetic basis of the diversity of behavioral responses exhibited in natural populations of many organisms, including humans. Furthermore, while mutagenic studies have been extremely successful in identifying genetic components for almost every behavioral trait of ecological interest, the methods often employed in these studies (knockout and large insertion/deletion mutants) are not likely to be representative of the type of variation present in natural populations (Boake et al. 2002). The data gathered here represent a more realistic picture of the distribution and timescale over which genetic and phenotypic variation in behavior can be expected to occur in natural populations and is therefore fundamental to our understanding of the mechanisms underlying the processes of both extinction and adaptation.
Acknowledgments
We thank J. Pierce-Shimomura and S. Lockery for software and experimental advice. We also thank J. Morphew and B. Gerami-Niani for their efforts in initial chemotaxis mutation accumulation experiments and anonymous reviewers for helpful comments. This work was supported by National Institutes of Health (NIH) grant GM54185 and National Science Foundation (NSF) grant DEB-0088083 to P.C.P., NIH grant GM36827 to M.L., and by training fellowships from the NSF (DBI-9413223) and the U.S. Public Health Service (GM-07413) to S.E.
References
- Azevedo, R. B., P. D. Keightley, C. Lauren-Maatta, L. L. Vassilieva, M. Lynch et al., 2002. Spontaneous mutational variation for body size in Caenorhabditis elegans. Genetics 162: 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann, C. I., 1998. Neurobiology of the Caenorhabditis elegans genome. Science 282: 2028–2033. [DOI] [PubMed] [Google Scholar]
- Bargmann, C. L., E. Hartweig and H. R. Horvitz, 1993. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74: 515–527. [DOI] [PubMed] [Google Scholar]
- Boake, C. R. B., S. J. Arnold, F. Breden, L. M. Meffert, M. G. Ritchie et al., 2002. Genetic tools for studying adaptation and the evolution of behavior. Am. Nat. 160: S143–S159. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, E. H., and D. H. Reed, 1989. Fitness decline under relaxed selection in captive populations. Conserv. Biol. 13: 665–669. [Google Scholar]
- Davies, E. K., A. D. Peters and P. D. Keightley, 1999. High frequency of cryptic deleterious mutations in Caenorhabditis elegans. Science 285: 1748–1751. [DOI] [PubMed] [Google Scholar]
- Estes, S., P. C. Phillips, D. R. Denver, W. K. Thomas and M. Lynch, 2004. Mutation accumulation in populations of varying size: the distribution of mutational effects for fitness correlates in Caenorhabditis elegans. Genetics 166: 1269–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes, S., B. C. Ajie, M. Lynch and P. C. Phillips, 2005. Spontaneous mutational correlations for life-history, morphological and behavioral characters in Caenorhabditis elegans. Genetics 170: 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke, J. J., R. B. Bass, S. L. Butler, S. A. Chervitz and M. A. Danielson, 1997. The two-component signaling pathway of bacterial chemotaxis: a molecular view of signal transduction by receptors, kinases, and adaptation enzymes. Annu. Rev. Cell Dev. Biol. 13: 457–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Dorado, A., and A. Gallego, 2003. Comparing analysis methods for mutation-accumulation data: a simulation study. Genetics 164: 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle, D., B. Morikawa and M. Lynch, 1996. Comparing mutational variabilities. Genetics 143: 1467–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovelin, R., and P. C. Phillips, 2005 Functional constraint and divergence in the G protein family in Caenorhabditis elegans and C. briggsae. Mol. Genet. Genomics (in press). [DOI] [PubMed]
- Jovelin, R., B. C. Ajie and P. C. Phillips, 2003. Molecular evolution and quantitative variation for chemosensory behaviour in the nematode genus Caenorhabditis. Mol. Ecol. 12: 1325–1337. [DOI] [PubMed] [Google Scholar]
- Keightley, P. D., 2004. Comparing analysis methods for mutation-accumulation data. Genetics 167: 551–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley, P. D., and A. Caballero, 1997. Genomic mutation rates for lifetime reproductive output and lifespan in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 94: 3823–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley, P. D., and M. Lynch, 2003. Toward a realistic model of mutations affecting fitness. Evolution 57: 683–685. [DOI] [PubMed] [Google Scholar]
- Keightley, P. D., and O. Ohnishi, 1998. EMS-induced polygenic mutation rates for nine quantitative characters in Drosophila melanogaster. Genetics 148: 753–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande, R., 1995. Mutation and conservation. Conserv. Biol. 9: 782–791. [Google Scholar]
- Lynch, M., and M. O'Hely, 2001. Supplementation and the genetic fitness of natural populations. Conserv. Genet. 2: 363–378. [Google Scholar]
- Lynch, M., and J. B. Walsh, 1998 Genetics and Analysis of Quantitative Traits. Sinauer Associates, Sunderland, MA.
- Lynch, M., J. Blanchard, D. Houle, T. Kibota, S. Schultz et al., 1999. Perspective: spontaneous deleterious mutation. Evolution 53: 645–663. [DOI] [PubMed] [Google Scholar]
- Pierce-Shimomura, J. T., T. M. Morse and S. R. Lockery, 1999. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J. Neurosci. 19: 9557–9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce-Shimomura, J. T., S. Faumont, M. R. Gaston, B. J. Pearson and S. R. Lockery, 2001. The homeobox gene lim-6 is required for distinct chemosensory representations in C. elegans. Nature 410: 694–698. [DOI] [PubMed] [Google Scholar]
- Prasad, B. C., and R. R. Reed, 1999. Chemosensation: molecular mechanisms in worms and mammals. Trends Genet. 15: 150–153. [DOI] [PubMed] [Google Scholar]
- Shabalina, S. A., L. Yampolsky and A. S. Kondrashov, 1997. Rapid decline of fitness in panmictic populations of Drosophila melanogaster maintained under relaxed natural selection. Proc. Natl. Acad. Sci. USA 94: 13034–13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, F. H., C. J. Geyer and R. G. Shaw, 2002. A comprehensive model of mutations affecting fitness and inferences for Arabidopsis thaliana. Evolution 56: 453–463. [DOI] [PubMed] [Google Scholar]
- Stadler, M., A. Mayer, H. Anke and O. Sterner, 1994. Fatty acids and other compounds isolated from cultures of Bascidiomycetes. Planta Med. 60: 128–132. [DOI] [PubMed] [Google Scholar]
- Troemel, E. R., 1999. Chemosensory signaling in C. elegans. BioEssays 21: 1011–1020. [DOI] [PubMed] [Google Scholar]
- Vassilieva, L. L., A. M. Hook and M. Lynch, 2000. The fitness effects of spontaneous mutations in Caenorhabditis elegans. Evolution 54: 1234–1246. [DOI] [PubMed] [Google Scholar]