Abstract
Marsupials present a series of genetic and chromosomal features that are highly conserved in very distant species. One of these features is the absence of a homologous region between X and Y chromosomes. According to this genetic differentiation, sex chromosomes do not synapse during the first meiotic prophase in males, and a special structure, the dense plate, maintains sex chromosome association. In this report we present results on the process of meiotic sex chromosome pairing obtained from three different species, Thylamys elegans, Dromiciops gliroides, and Rhyncholestes raphanurus, representing the three orders of American marsupials. We have investigated the relationships between the axial structures organized along sex chromosomes and the formation of the dense plate. We found that in the three species the dense plate arises as a modification of sex chromosomal axial elements, but without the involvement of other meiotic axial structures, such as the cohesin axes. Considering the phylogenetic relationships among the marsupials studied here, our data reinforce the idea that the dense plate emerged early in marsupial evolution as an efficient mechanism to ensure the association of the nonhomologous sex chromosomes. This situation could have influenced the further evolution of sex chromosomes in marsupials.
THE absence of a region of homology between X and Y sex chromosomes in marsupials (Graves and Watson 1991) is one of the most remarkable genetic features of this group of mammals. This lack of homology is thought to have been originated by the regional suppression of recombination between the proto X and the proto Y, caused by a chromosomal rearrangement and/or as a mechanism to protect the association of sex-determining genes (Solari 1993; Graves 1995). In the absence of recombination, the Y chromosome becomes genetically isolated and undergoes a gradual process of genetic differentiation and degeneration by the accumulation of deleterious mutations that eventually become fixed in the populations through stochastic and/or selective forces (Maynard Smith and Haigh 1974; Charlesworth 1991; Rice 1996; Bachtrog 2004).
The genetic differentiation of sex chromosomes poses a serious problem for these chromosomes to perform a proper meiotic behavior. In eutherian mammals pairing and segregation of X and Y chromosomes are ensured by the presence of distal homologous regions, called pseudoautosomal regions, or PARs (Burgoyne 1982), in which both synapsis and recombination occur (Solari 1974). The formation of a chiasma in the PAR holds sex chromosomes associated until they segregate at anaphase I. Accordingly, the presence of the PAR is essential for male fertility in mice (Burgoyne et al. 1992) and humans (Mohandas et al. 1992). However, marsupial sex chromosomes do not have PAR, and this determines the meiotic structure and behavior of these chromosomes. Thus, during the first meiotic prophase, sex chromosomes pair, in the sense that they appear associated in the nucleus, but they do not synapse; i.e., there is no formation of a synaptonemal complex (SC) central element (CE) between their axial elements (AEs). Instead, a specific structure called dense plate (DP) appears associating the ends of sex chromosomal AEs (Solari and Bianchi 1975; Sharp 1982; Roche et al. 1986; Seluja et al. 1987). The DP maintains sex chromosome association at least up to the end of the pachytene stage of the first meiotic prophase. On these grounds (absence of PAR and synapsis), recombination should not occur between marsupial X and Y chromosomes.
In a recent report, we showed that in the American marsupial Thylamys elegans the SCP3 protein, a structural component of the lateral elements (LEs) of the SC in mammals (Dobson et al. 1994; Lammers et al. 1994), is also one of the components of the DP (Page et al. 2003). These results strongly indicate that the formation of the DP in this species occurs as a modification of the sex chromosomal AEs. However, to what extent these results are valid for other marsupials has not been addressed.
In this article we report a comparative study of male meiosis in three marsupial species that represent the three extant orders of American marsupials: T. elegans (Didelphimorphia), Dromiciops gliroides (Microbiotheria), and Rhyncholestes raphanurus (Paucituberculata). The study of the two last species is especially valuable because D. gliroides is the only extant member of Microbiotheria, while R. raphanurus is one of the seven extant species of Paucituberculata. We have analyzed the involvement of the axial structures organized along sex chromosomes in the formation of the DP. For this purpose we focused on the localization of SCP3 and SCP1 proteins of the SC. Recently, a new element has emerged as an important organizer of the meiotic chromosome structure: the cohesin axis (CA) (Pelttari et al. 2001; Prieto et al. 2001, 2004), in which a wide range of proteins are involved, including SMC1α, SMC1β, SMC3, STAG3, REC8, and RAD21 (Eijpe et al. 2000, 2003; Prieto et al. 2001; Revenkova et al. 2001; Parra et al. 2004). In this article we also investigate the possible role of STAG3 and SMC3 cohesins in the organization of the DP. We complete our analysis with the localization of phosphoproteins recognized by the MPM-2 antibody, which has proved to be a specific marker of the sex chromosomal AEs and the DP in T. elegans. Our results show that the structure, molecular composition, and meiotic behavior of sex chromosomes in the three species is very similar, indicating an early origin of such features in marsupial sex chromosome evolution.
MATERIALS AND METHODS
Males of T. elegans Waterhouse (Didelphidae) were collected from wild populations in the central region of Chile. Males of D. gliroides Philippi (Microbiotheridae) and R. raphanurus Osgood (Caenolestidae) were collected in the Vicente Pérez Rosales National Park with permission of the Corporación Nacional Forestal. An additional male of D. gliroides, collected in the locality of Temuco, was kindly provided by Eduardo Palma (Santiago, Chile). The specimens were castrated by a cut at the base of the scrotum, and the seminiferous tubules were extracted and fixed for further processing.
Immunofluorescence:
The seminiferous tubules were either squashed or spread following the procedures described by Page et al. (1998) and Peters et al. (1997), respectively. The slides were incubated with the following primary antibodies diluted in PBS: a rabbit serum that recognizes the SCP3 protein of the SC lateral elements (Lammers et al. 1994) at a 1:500 dilution; a rabbit serum that recognizes the SCP1 protein of the SC central element (Meuwissen et al. 1992) at a 1:200 dilution; the monoclonal MPM-2 antibody (Upstate, Lake Placid, NY), which recognizes phosphoepitopes at a 1:1000 dilution; a rabbit serum that recognizes the STAG3 protein of the cohesin complex (Prieto et al. 2001) at a 1:25 dilution; a rabbit serum that recognizes the SMC3 protein (AB3914, Chemicon, Temecula, CA) raised against a synthetic peptide from human SMC3 at a 1:30 dilution; and a human anticentromere serum that recognizes centromeric proteins at a 1:100 dilution. The incubations were carried out for 1 hr at room temperature in a moist chamber. Then the slides were rinsed in PBS three times for 5 min each and incubated for 45 min at room temperature with the appropriate secondary antibodies: fluorescein-isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (Jackson) at a 1:100 dilution, Texas red (TR)-conjugated goat anti-rabbit IgG (Jackson) at a 1:150 dilution, TR-conjugated goat anti-mouse IgG (Jackson) at a 1:100 dilution, and TR-conjugated goat anti-human IgG (Jackson) at a 1:150 dilution. The slides were rinsed three times for 5 min each in PBS and stained with 2 μg/ml DAPI. After a final rinse in PBS, the slides were mounted with Vectashield (Vector, Burlingame, CA). For double detection of two antibodies raised in rabbit, we followed the procedure described in Page et al. (2003).
Observations were made on either a Nikon Optiphot or an Olympus BX61 microscope equipped with epifluorescence optics and the images were photographed on Fujichrome Provia 400F or captured with an Olympus DP70 CCD camera. Images were processed with Adobe Photoshop 7 and ImageJ softwares.
RESULTS
Pairing of sex chromosomes:
To accurately analyze the process of sex chromosome approach and pairing during first meiotic prophase, it is important to keep the native position of the chromosomes within the nucleus. To do this, we have employed a squashing technique that maintains the three-dimensional organization of the cell (Page et al. 1998).
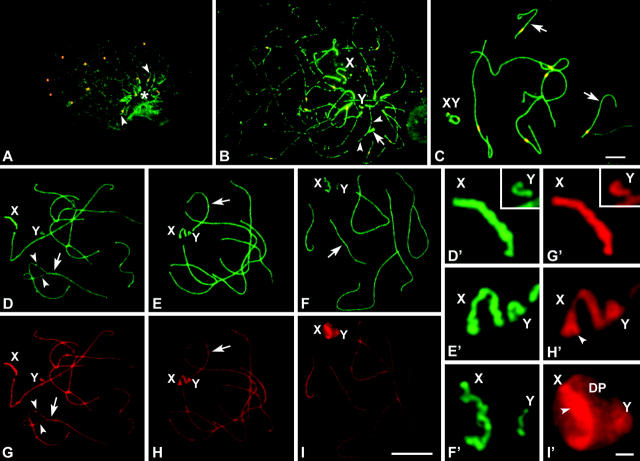
We first analyzed the distribution of SC proteins. Sex chromosomes form AEs that are labeled with anti-SCP3. These AEs remain located at the nuclear periphery through the first meiotic prophase, but their morphology and relative position change as prophase proceeds. Thus, during zygotene (Figure 1, A and A′) and early pachytene (Figure 1, B and B′) sex chromosomal AEs appear as thick lines strongly labeled with anti-SCP3 (especially in R. raphanurus; see Figure 2, D–I, and Figure 4), and both sex chromosomes are clearly separated, occupying very distant domains in the nuclear periphery. However, in midpachytene, sex chromosomes approach and associate with each other. At this time, their AEs become thinner and SCP3 fades along them (Figure 1, C and C′). Furthermore, a structure labeled with anti-SCP3 appears in the region of attachment of sex chromosomal AEs to the nuclear envelope. This structure is the DP, which starts to develop at the time of sex chromosome pairing in midpachytene and remains bound to sex chromosomes at least until spermatocytes enter in the diplotene or the diffuse stage (Figure 1, D and D′). In D. gliroides we observed the presence of a DP in each sex chromosome separately in some pachytene spermatocytes (not shown). This situation is exclusive to this species and seems to be transient, since all diplotene spermatocytes showed sex chromosomes paired and associated with a single DP.
Figure 1.—
Immunolabeling of D. gliroides squashed spermatocytes with anti-SCP3 (green) and anticentromere (red) antibodies. Several optical planes of each nucleus were taken and subsequently superimposed. Only partial reconstructions of the nuclei are shown. (A) Zygotene. SCP3 appears on the autosomes as solid lines (arrow), whose ends are polarized toward a region of the nucleus (asterisk). Most autosomes are already synapsed. Sex chromosomes (X, Y) have well-defined AEs, but they appear located in opposite places in the bouquet area. (A′) Detail of the sex chromosomal AEs. (B and B′) Early pachytene. All autosomes are fully synapsed (arrow), but sex chromosomes still remain separated to each other in the nucleus, presenting thick and stiff AEs. (C and C′) Mid-late pachytene. Autosomes remain synapsed (arrow) and sex chromosomes are associated. The sex chromosomal AEs appear thinner than in previous stages and their labeling with anti-SCP3 is fainter. The DP labeled with anti-SCP3 is located on the tips of both sex chromosomal AEs. (D and D′) Diplotene. Autosomes desynapse and their LEs (arrowheads) appear separated all along the chromosome except at certain points (arrow). Sex chromosomes remain associated and the DP appears still organized. Bars: 5 μm for A–D; 1 μm for A′–D′.
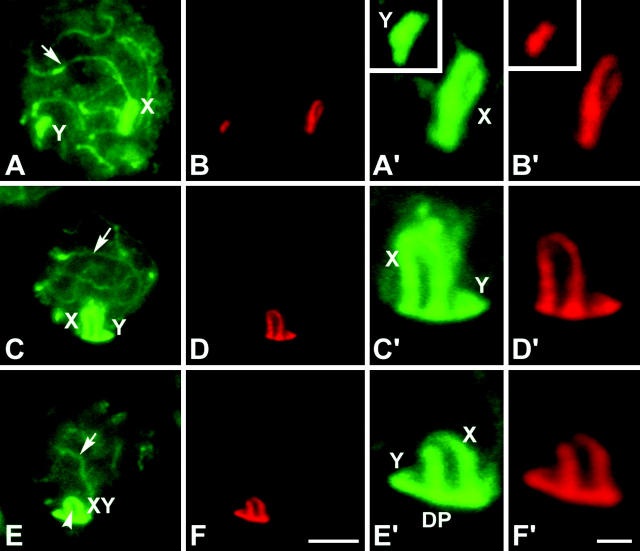
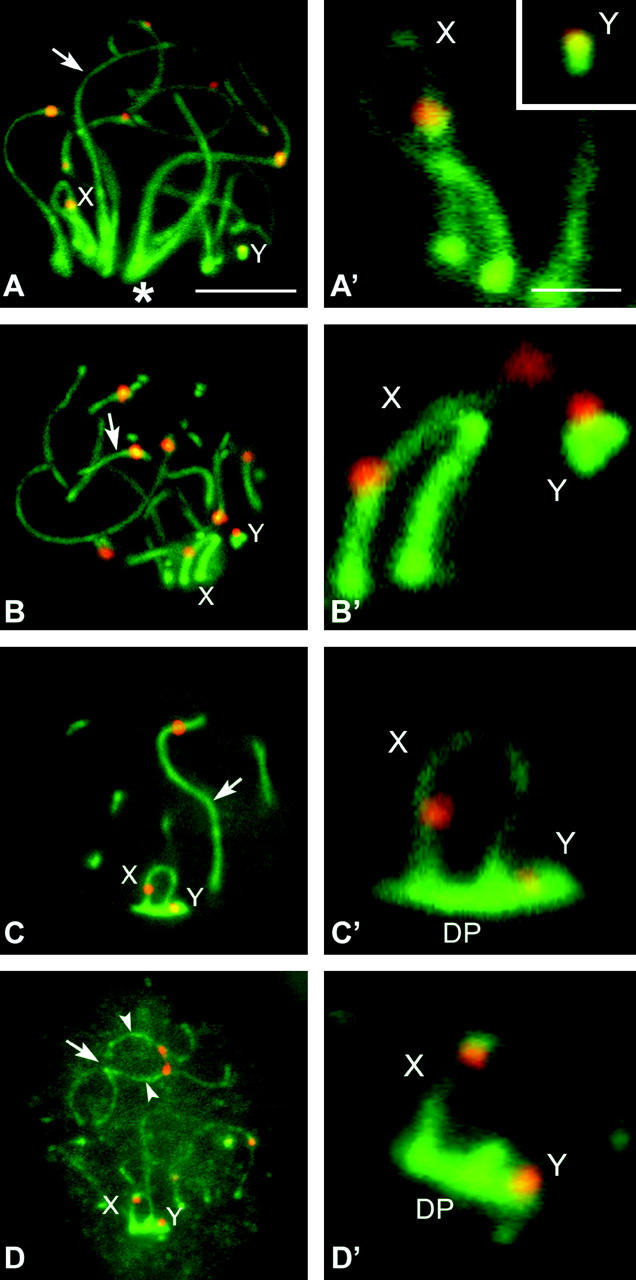
Figure 2.—
(A–C) Immunolabeling of spread spermatocytes of T. elegans with anti-STAG3 (green) and anticentromere antibodies (red). (A) Leptotene. STAG3 is present as small dots evenly distributed in the whole nucleus. These dots seem to condense to form linear structures (arrowheads) in a specific region (asterisk). All centromeres appear unpaired. (B). Zygotene. STAG3 appears forming irregular and interrupted linear structures. These lines are individualized in most of their length, but associate in pairs in some regions (arrow) and form fork-like structures. STAG3 appears in sex chromosomes (X, Y) as regular lines of similar thickness to the synapsed regions of autosomes. (C) Mid-late pachytene. The cohesin axes of autosomes are fully associated. Sex chromosomes appear associated, showing a regular labeling with STAG3, but there is no connection between the axes of the X and Y chromosomes. (D–I′) Immunolabeling of R. raphanurus spread spermatocytes with anti-STAG3 (green) and anti-SCP3 (red) antibodies. (D) Late zygotene. Autosomes are almost completely synapsed (arrow) and only some regions are still unsynapsed (arrowheads). Sex chromosomes (X, Y) lie apart from each other. (G) The same nucleus after SCP3 labeling. The localization of STAG3 and SCP3 is identical in both the synapsed and the unsynapsed regions of autosomes and sex chromosomes (enlarged in D′ and G′). (E and H) Midpachytene. Autosomes are fully synapsed and both proteins have a similar localization. However, the distribution of STAG3 and SCP3 is different on the sex chromosomes. STAG3 shows a linear pattern on both sex chromosomes, but this axial structure presents an irregular outline (E′). The outlines of the axes revealed by SCP3 are more regular and expand at the tips of both the X and Y chromosomes. (F and I) Late pachytene. STAG3 (F) and SCP3 (I) colocalize on the autosomes but present different patterns in sex chromosomes. While STAG3 reveals linear structures on the X and Y (F′), SCP3 not only reveals these axial structures but also appears in a plate-like structure in which sex chromosomal AEs are immersed (I′). This structure is the DP. Bars: 5 μm for A–C; 10 μm for D–I; 1 μm for D′–I′.
Figure 4.—
Immunolabeling of R. raphanurus squashed spermatocytes with anti-SCP3 (green) and MPM-2 (red). Single focal planes are shown. SCP3 labels both autosomal LEs (arrows) and sex chromosomal AEs (X, Y). However, MPM-2 antibody appears only on the AEs of sex chromosomes and on the dense plate. (A and B) Early pachytene. Sex chromosomes occupy different domains in the nucleus. (A′ and B′) Detail of the sex chromosomes. Note that the labeling of both antibodies is almost identical. (C–D′) Midpachytene. Sex chromosomes have approached and start to contact by the ends of their AEs. (E–F′). Late pachytene. Sex chromosomes are paired and the DP has formed. Bars: 5 μm for A–F; 1 μm for A′–F′.
The immunolocalization of SCP1 (the main component of the SC central element) revealed that this protein does not appear to mediate the association of sex chromosomal AEs in either of these species (data not shown), thus indicating the absence of tripartite SC.
The dense plate does not contain components of the cohesin complex:
To determine whether some components of the cohesin complex were involved in the formation of the DP, we analyzed the localization of the STAG3 and SMC3 proteins. STAG3, a member of the cohesin complex exclusively expressed in meiosis, is first detected in spermatocytes during leptotene as discrete spots (Figure 2A). In zygotene these spots line up to form individual threads. The CAs resemble the trajectories of the chromosomal AEs in both autosomes and sex chromosomes. Moreover, they congregate in a specific region of the nucleus (the bouquet area) and some of the filaments appear associated in a similar way to synapsing AEs (Figure 2B). This process culminates with the intimate association of the CAs along the autosomal bivalents during pachytene (Figure 2C). To determine whether the axial structures revealed with anti-STAG3 were associated with the AEs and LEs of the SC, we performed a double immunolocalization of SCP3 and STAG3 in T. elegans and R. raphanurus (Figure 2, D–I). We found that in fact both proteins follow the same cycle of assembly and that they colocalize on the autosomes.
STAG3 also reveals axial structures on the sex chromosomes, whose CAs start to differentiate in zygotene (Figure 2B) and remain clearly differentiated during pachytene (Figure 2C). As for autosomes, we compared the localization of STAG3 and SCP3 on the sex chromosomes. We found that in zygotene the location of both proteins is very similar. The axial structures (AEs and CAs) of the X and Y chromosomes appear thickened and both signals are almost completely coincident (Figure 2, D and D′ and G and G′). However, as cells progress into pachytene and sex chromosomes approach each other in the nucleus, the distribution pattern of both proteins starts to show differences. While SCP3 begins to accumulate on the region of sex chromosome attachment to the nuclear periphery (Figure 2, H and H′), STAG3 does not (Figure 2, E and E′). This broadening of the sex chromosomal AEs revealed with anti-SCP3 represents the first step of the formation of the DP. This difference becomes more evident in mid-late pachytene, once the DP is formed. SCP3 labels both the AEs of the sex chromosomes and the DP, while STAG3 appears depicting only the trajectory of the sex CAs, which do not show any structural modification (Figure 2, F and F′ and I and I′).
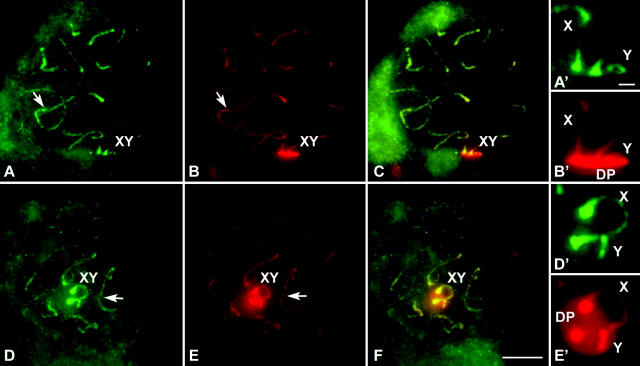
We have also analyzed the distribution of SMC3, a constitutive member of the cohesin complex expressed in both mitosis and meiosis (Eijpe et al. 2000). SMC3 shows a cycle of appearance and location in both autosomes and sex chromosomes similar to that of STAG3; i.e., SMC3 is present along the sex chromosomes forming an axial structure, but is completely absent from the DP (Figure 3). Three-dimensional reconstructions of the sex body show the DP as a flat structure labeled with SCP3 in which the axes (both AEs and CAs) are immersed (see supplementary movies 1 and 2 at http://www.genetics.org/supplemental/).
Figure 3.—
Immunolabeling of two pachytene squashed spermatocytes of T. elegans with anti-SMC3 antibodies (green) and anti-SCP3 (red). Several optical planes were taken of each nucleus and subsequently superimposed. (A) SMC3 is present along autosomes (arrow) as lines depicting the whole length of bivalents and is also present on the sex chromosomal axial elements (XY). (A′) Enlargement of the sex chromosomes. (B) SCP3 show the same pattern of distribution except for the sex chromosomes (XY), in which an intense labeling is detected in the region of association to the nuclear periphery, corresponding to the DP. (B′) Enlargement of the sex chromosomes. The DP is seen in lateral view. (C) Merge of SMC3 and SCP3 labeling. (D–F) A different spermatocyte, in which the DP is seen in polar view. Sex chromosomes are enlarged in D′ and E′. Bars: 5 μm for A–F; 1 μm for A′–E′.
Specific phosphorylation of sex chromosomal AEs:
The existence of specific modifications of sex chromosome structure during first meiotic prophase was studied by simultaneous immunolabeling of SCP3 and MPM-2, an antibody that recognizes a wide range of mitotic phosphoproteins (Davis et al. 1983; Renzi et al. 1997) and that has been previously reported to label the sex chromosomal AEs and the DP in T. elegans (Page et al. 2003). We found that the labeling of MPM-2 antibody during first meiotic prophase is also exclusively located on the sex chromosomes in D. gliroides and R. raphanurus. This labeling appears as lines that depict the trajectories of sex chromosomal AEs during zygotene (not shown) and early pachytene (Figure 4, A–D′). In addition, MPM-2 is alsolocated in the DP, which appears in the region of attachment of the sex chromosomes to the nuclear periphery in late pachytene (Figure 4, E–F′). Double immunolabeling shows a complete correspondence in the location of SCP3 and MPM-2 on the sex chromosomes throughout the first meiotic prophase. These results indicate that MPM-2 recognizes a phosphoprotein present on the AEs of sex chromosomes, and also on the DP, but not on the autosomal bivalents. Furthermore, given the differences shown by AEs and CAs, it is likely that this phosphorylation is not related to modifications of the cohesin complex proteins. The phosphorylation of sex chromosomal AEs indicates that sex chromosome structure is specifically regulated by the phosphorylation of some of their components and/or by the presence of specific proteins. The identification of such components would add valuable information about their plausible role on the regulation of sex chromosome structure and behavior during marsupial meiosis.
DISCUSSION
The dense plate derives from elements of the synaptonemal complex but it does not involve components of the cohesin complex:
Ultrastructural studies have shown that the meiotic behavior of marsupial sex chromosomes deviates from that of most eutherian species (Solari and Bianchi 1975; Sharp 1982; Roche et al. 1986; Seluja et al. 1987). Rather than a region of synapsis, the marsupial X and Y are held together during first meiotic prophase by the specialized structure DP (Solari and Bianchi 1975). Although ultrastructural studies have elucidated the morphological organization of the DP, we provide new information on the protein components involved in the formation of this structure. We found that SCP3 and the cohesin subunits STAG3 and SMC3 show a similar, but not identical, location and expression in marsupial meiosis. The most relevant difference is the participation of SCP3, but not STAG3 or SMC3, in the formation of the DP. This means that the special mode of sex chromosome pairing relies on modifications of some of the structural components of the AEs, indicating a remarkable plasticity of this structure in marsupial meiosis.
Our results also bring new information on the dynamics and relationships between the axial structures that are organized along meiotic chromosomes. Pelttari et al. (2001) have reported the persistence of the CAs in spermatocytes defective for the formation of the AEs, indicating that AEs and CAs are in fact different structures. This hypothesis is reinforced by the finding that in mouse and human females, AEs and CAs do not show the same location in some meiotic stages (Prieto et al. 2004). The location of AE and CA proteins on the sex chromosomes in male marsupials is consistent with this model. Therefore, AEs and CAs should be considered as different structures, and more significantly, they could be regulated in different ways since both structures are able to follow a different pattern of development on the sex chromosomes during first meiotic prophase. This is noteworthy because it has been claimed that, in the absence of SCP3, cohesin proteins may perform some of the functions attributed to the AEs, such as the recruitment of components of the CE of the SC and the recombination machinery (Pelttari et al. 2001). However, AEs may play additional roles during male meiosis that cannot be performed completely by other components. The morphological modification of sex chromosomal AEs to form the marsupial DP may be an example of such roles.
The meiotic program of sex chromosome pairing in marsupials presents modifications that are highly conserved in very distant species:
The three species studied here present a similar pattern of sex chromosome behavior in agreement with previous reports, which indicated that the structural modification of sex chromosomal AEs, the delay in their pairing, and the formation of the DP are common to a wide range of marsupials, including 3 American (Solari and Bianchi 1975; Roche et al. 1986; Seluja et al. 1987) and 22 Australian species (Sharp 1982). We show here that this common behavior may include other interesting features, such as the specific phosphorylation of sex chromosomal AE components and the origin of the DP as a modification of the AEs, but not the CAs. Thus, sex chromosomes in marsupials display a complex and specific program of pairing, which appears as an orderly succession of events that lead to the ultimate association of sex chromosomes in the prophase I nucleus. This program is quite different from pairing of autosomes and also of sex chromosomes of eutherian mammals, in which pairing would be induced by homology-search mechanisms (Mahadevaiah et al. 2001).
A series of morphological and genetic studies have revealed that the orders Didelphimorphia and Paucituberculata to which T. elegans and R. raphanurus, respectively, belong, are the most basal groups within marsupials (Szalay 1994; Amrine-Madsen et al. 2003; Nilsson et al. 2003). As for D. gliroides, it has been repeatedly claimed that, in spite of its South American distribution, this species is more closely related to Australian marsupials (Kirsch et al. 1991; Szalay 1994; Spotorno et al. 1997; Palma and Spotorno 1999; Janke et al. 2002; Amrine-Madsen et al. 2003; Nilsson et al. 2003). Taken together, such relationships give support to the idea that the special features that sex chromosomes present during meiosis could have originated very early in the evolution of marsupials [stemming at least from the radiation of the extant marsupial orders 64 million years ago (Nilsson et al. 2003)], and they would have been maintained almost invariably throughout the subsequent evolution and radiation of this group.
The dense plate and the evolution of sex chromosomes in marsupials:
Marsupial sex chromosomes are the smallest among mammals (Sharman 1973; Hayman 1990; Graves 1995, 1996; Toder et al. 2000). This fact points out that the genetic erosion of the Y chromosome may have been more drastic in marsupials than in other mammalian groups. Moreover, the genetic content of the Y chromosome seems to be dispensable in the somatic line in some marsupial species (Hayman 1990; Palma 1995), including D. gliroides (Gallardo and Paterson 1987). As mentioned above, the absence of recombination poses serious problems for sex chromosomes undergoing a proper meiosis and promotes the genetic degradation of the Y chromosome (Rice 1996). In eutherians, the successive acquisitions of PARs during sex chromosome evolution solved the problem of meiotic segregation and also counterbalanced, at least in part, the genetic degradation of the Y chromosome (Graves 1995). On the other hand, the problem of pairing was solved in marsupials through the development of the DP, but this mechanism kept the Y chromosome genetically isolated. One could speculate that the special pairing mode of sex chromosomes in marsupials, while ensuring their cytological transmission, could have reinforced the isolation of the Y chromosome and, hence, its degradation.
Acknowledgments
We express our sincere thanks to Juan Luis Santos and Carlos García de la Vega for their critical reading of the manuscript, Christa Heyting (Wageningen, The Netherlands) for providing the SCP1 and SCP3 antibodies, and Chantal Andre (Hospital Henry Mondor, Paris) for the anticentromere serum. We are also indebted to Juan Oyarce for technical assistance in the field and in the laboratory, to Eduardo Palma (Santiago, Chile) for the gift of one specimen of D. gliroides, and to Servicio Agrícola y Ganadero and Corporación Nacional Forestal for collecting permissions. This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) grants 2000008, 1040910, and 7040174 (Chile); grant BMC2002-00043 from Ministerio de Ciencia y Tecnología (Spain); and a grant from Centro de Estudios de América Latina-Banco Santander Central Hispano. J.P. was a Mutis fellowship of the Agencia Española de Cooperación Internacional (AECI), M.T.P. was supported by a fellowship from Fundación Ramón Areces, and A.V. received support from Fundación General de la Universidad Autónoma de Madrid (FGUAM) and Olympus Optical España S.A.
References
- Amrine-Madsen, H., M. Scally, M. Westerman, M. J. Stanhope, C. Krajewski et al., 2003. Nuclear gene sequences provide evidence for the monophyly of australidelphian marsupials. Mol. Phylogenet. Evol. 28: 186–196. [DOI] [PubMed] [Google Scholar]
- Bachtrog, D., 2004. Evidence that positive selection drives Y-chromosome degeneration in Drosophila miranda. Nat. Genet. 36: 518–522. [DOI] [PubMed] [Google Scholar]
- Burgoyne, P. S., 1982. Genetic homology and crossing over in the X and Y chromosomes of mammals. Hum. Genet. 61: 85–90. [DOI] [PubMed] [Google Scholar]
- Burgoyne, P. S., S. K. Mahadevaiah, M. J. Sutcliffe and S. J. Palmer, 1992. Fertility in mice requires X-Y pairing and a Y-chromosomal “spermiogenesis” gene mapping to the long arm. Cell 71: 391–398. [DOI] [PubMed] [Google Scholar]
- Charlesworth, B., 1991. The evolution of sex chromosomes. Science 251: 1030–1033. [DOI] [PubMed] [Google Scholar]
- Davis, F. M., T. Y. Tsao, S. K. Fowler and P. N. Rao, 1983. Monoclonal antibodies to mitotic cells. Proc. Natl. Acad. Sci. USA 80: 2926–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, M. J., R. E. Pearlman, A. Karaiskakis, B. Spyropoulos and P. B. Moens, 1994. Synaptonemal complex proteins: occurrence, epitope mapping and chromosome disjunction. J. Cell Sci. 107: 2749–2760. [DOI] [PubMed] [Google Scholar]
- Eijpe, M., C. Heyting, B. Gross and R. Jessberger, 2000. Association of mammalian SCM1 and SCM3 proteins with meiotic chromosomes and synaptonemal complex. J. Cell Sci. 113: 673–682. [DOI] [PubMed] [Google Scholar]
- Eijpe, M., H. Offenberg, R. Jessberger, E. Revenkova and C. Heyting, 2003. Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1beta and SMC3. J. Cell Biol. 160: 657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo, M. H., and B. D. Paterson, 1987. An additional 14-chromosome karyotype and sex-chromosome mosaicism in South American marsupials. Fieldiana Zool. 39: 111–116. [Google Scholar]
- Graves, J. A. M., 1995. The origin and function of the mammalian Y chromosome and Y-borne genes—an evolving understanding. BioEssays 17: 311–321. [DOI] [PubMed] [Google Scholar]
- Graves, J. A. M., 1996. Mammals that break the rules: genetics of marsupials and monotremes. Annu. Rev. Genet. 30: 233–260. [DOI] [PubMed] [Google Scholar]
- Graves, J. A. M., and J. M. Watson, 1991. Mammalian sex chromosomes: evolution of organization and function. Chromosoma 101: 63–68. [DOI] [PubMed] [Google Scholar]
- Hayman, D. L., 1990. Marsupial cytogenetics. Aust. J. Zool. 37: 331–349. [Google Scholar]
- Janke, A., O. Magnell, G. Wieczorek, M. Westerman and U. Arnason, 2002. Phylogenetic analysis of 18S rRNA and the mitochondrial genomes of the wombat, Vombatus ursinus, and the spiny anteater, Tachyglossus aculeatus: increased support for the marsupionta hypothesis. J. Mol. Evol. 54: 71–80. [DOI] [PubMed] [Google Scholar]
- Kirsch, J. A., A. W. Dickerman, O. A. Reig and M. S. Springer, 1991. DNA hybridization evidence for the Australian affinity of the American marsupial Dromiciops australis. Proc. Natl. Acad. Sci. USA 88: 10465–10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers, J. H. M., H. H. Offenberg, M. van Aalderen, A. C. G. Vink, A. J. J. Dietrich et al., 1994. The gene encoding a major component of the lateral elements of synaptonemal complexes of the rat is related to X-linked lymphocyte-regulated genes. Mol. Cell. Biol. 14: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah, S. K., J. M. A. Turner, F. Baudat, E. P. Rogakou, P. de Boer et al., 2001. Recombinational DNA double-strand breaks in mice precede synapsis. Nat. Genet. 27: 271–276. [DOI] [PubMed] [Google Scholar]
- Maynard Smith, J., and J. Haigh, 1974. The hitch-hiking effect of a favorable gene. Genet. Res. 23: 23–35. [PubMed] [Google Scholar]
- Meuwissen, R. L. J., H. H. Offenberg, A. J. J. Dietrich, A. Riesewijk, M. van Iersen et al., 1992. A coiled-coil related protein specific for synapsed regions of the meiotic prophase chromosomes. EMBO J. 11: 5091–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas, T. K., R. M. Speed, M. B. Passage, P. H. Yen, A. C. Chandley et al., 1992. Role of the pseudoautosomal region in sex chromosome pairing during male meiosis: meiotic studies in a man with a deletion of distal Xp. Am. J. Hum. Genet. 51: 526–533. [PMC free article] [PubMed] [Google Scholar]
- Nilsson, M., A. Gullberg, A. E. Spotorno, U. Arnason and A. Janke, 2003. Radiation of marsupials after the K/T boundary: evidence from complete mitochondrial genomes. J. Mol. Biol. 57: 1–10. [DOI] [PubMed] [Google Scholar]
- Page, J., J. A. Suja, J. L. Santos and J. S. Rufas, 1998. Squash procedure for protein immunolocalization in meiotic cells. Chromosome Res. 6: 639–642. [DOI] [PubMed] [Google Scholar]
- Page, J., S. Berríos, J. S. Rufas, M. T. Parra, J. A. Suja et al., 2003. The pairing of X and Y chromosomes during meiotic prophase in the marsupial species Thylamys elegans is maintained by a dense plate developed from their axial elements. J. Cell Sci. 116: 551–560. [DOI] [PubMed] [Google Scholar]
- Palma, R. E., 1995. The karyotypes of two South American mouse opossums of the genus Thylamys (Marsupialia: Didelphidae), from the Andes and eastern Paraguay. Proc. Biol. Soc. Washington. 108: 1–5. [Google Scholar]
- Palma, R. E., and A. E. Spotorno, 1999. Molecular systematics of marsupials based on the rRNA 12S mitochondrial gene: the phylogeny of Didelphimorphia and of the living fossil microbiotheriid Dromiciops gliroides Thomas. Mol. Phylogenet. Evol. 13: 525–535. [DOI] [PubMed] [Google Scholar]
- Parra, M. T., A. Viera, R. Gomez, J. Page, R. Benavente et al., 2004. Involvement of the cohesin Rad21 and SCP3 in monopolar attachment of sister kinetochores during mouse meiosis-I. J. Cell Sci. 117: 1221–1234. [DOI] [PubMed] [Google Scholar]
- Pelttari, J., M-R. Hoja, L. Yuan, J-G. Liu, E. Brundell et al., 2001. A meiotic chromosomal core consisting of cohesin complex proteins recruits DNA recombination proteins and promotes synapsis in the absence of an axial element in mammalian meiotic cells. Mol. Cell. Biol. 21: 5667–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, A. H. F. M., A. W. Plug, M. J. van Vugt and P. de Boer, 1997. A drying-down technique for the spreading of mammalian meiocytes from the male and female germ line. Chromosome Res. 5: 66–71. [DOI] [PubMed] [Google Scholar]
- Prieto, I., J. A. Suja, N. Pezzi, L. Kremer, C. Martínez-A et al., 2001. Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis-I. Nat. Cell Biol. 3: 761–766. [DOI] [PubMed] [Google Scholar]
- Prieto, I., C. Tease, N. Pezzi, J. M. Buesa, S. Ortega et al., 2004. Cohesin component dynamics during meiotic prophase-I in mammalian oocytes. Chromosome Res. 12: 197–213. [DOI] [PubMed] [Google Scholar]
- Renzi, L., M. S. Gersch, M. S. Campbell, L. Wu, S. A. Osmani et al., 1997. MPM-2 antibody-reactive phosphorylations can be created in detergent-extracted cells by kinetochore-bound and soluble kinases. J. Cell Sci. 110: 2013–2025. [DOI] [PubMed] [Google Scholar]
- Revenkova, E., M. Eijpe, C. Heyting, B. Gross and R. Jessberger, 2001. Novel meiosis-specific isoform of mammailan SMC1. Mol. Cell. Biol. 21: 6984–6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, W. R., 1996. Evolution of the Y sex chromosome in animals. Bioscience 46: 331–343. [Google Scholar]
- Roche, L., G. Seluja and R. Wettstein, 1986. The meiotic behaviour of the XY pair in Lutreolina crassicaudata (Marsupialia: Didelphoidea). Genetica 71: 213–224. [Google Scholar]
- Seluja, G., L. Roche and A. J. Solari, 1987. Male meiotic prophase in Didelphis albiventris. J. Hered. 78: 218–222. [Google Scholar]
- Sharman, G. B., 1973 The chromosomes of non-eutherian mammals, pp. 485–530 in Cytotaxonomy and Vertebrate Evolution, edited by A. B. Chiarelli and E. Capanna. Academic Press, London.
- Sharp, P., 1982. Sex chromosome pairing during male meiosis in marsupials. Chromosoma 86: 27–47. [DOI] [PubMed] [Google Scholar]
- Solari, A. J., 1974. The behavior of the XY pair in mammals. Int. Rev. Cytol. 38: 273–317. [DOI] [PubMed] [Google Scholar]
- Solari, A. J., 1993 Sex Chromosomes and Sex Determination in Vertebrates. CRC Press, Boca Raton, FL.
- Solari, A. J., and N. O. Bianchi, 1975. The synaptic behaviour of the X and Y chromosomes in the marsupial Monodelphis dimidiata. Chromosoma 52: 11–25. [DOI] [PubMed] [Google Scholar]
- Spotorno, A. E., J. C. Marín, M. Yévenes, L. I. Walker, R. Fernández-Donoso et al., 1997. Chromosome divergences among American marsupials and the Australian affinities of the American Dromiciops. J. Mammal. Evol. 4: 259–269. [Google Scholar]
- Szalay, F. S., 1994 Evolutionary History of the Marsupials and an Analysis of Osteological Characters. Cambridge University Press, Cambridge, UK.
- Toder, R., M. J. Wakefield and J. A. Graves, 2000. The minimal mammalian Y chromosome—the marsupial Y as a model system. Cytogenet. Cell Genet. 91: 285–292. [DOI] [PubMed] [Google Scholar]