Abstract
A general MHC-heterozygote advantage in parasite-infected organisms is often assumed, although there is little experimental evidence for this. We tested the response of MHC-congenic mice (F2 segregants) to malaria and found the course of infection to be significantly influenced by MHC haplotype, parasite strain, and host gender. However, the MHC heterozygotes did worse than expected from the average response of the homozygotes.
WHEN fighting infection, MHC heterozygotes are often expected to be superior to both their respective homozygotes (i.e., exhibit overdominance) because they can present a wider range of antigens to T lymphocytes (Doherty and Zinkernagel 1975). Indeed, MHC heterozygotes normally show slower disease progression and/or more rapid clearance of an infection than the average of homozygotes, further providing a population-level advantage of heterozygosity (e.g., Thursz et al. 1997; Carrington et al. 1999), and MHC heterozygotes are typically overrepresented in vertebrate populations (e.g., Hedrick and Thomson 1983; Black and Hedrick 1997). However, in population studies it is often unclear whether the observed heterozygote advantage is due to overdominance, to dominance of resistance, or explained only by the specific allele frequencies in a host population (Lipsitch et al. 2003). Allele-specific measures are therefore necessary to explain the kind of heterozygote advantage that is observed (Apanius et al. 1997; Penn et al. 2002; McClelland et al. 2003).
We studied MHC-congenic mice during experimental exposure to two clones of Plasmodium chabaudi. F2 segregants were used as hosts (Ntotal = 107) to compare different homozygotes and the respective heterozygotes and to control for possible maternal effects or differences in the background genetics among congenic strains (see below). We used a fully factorial experimental design to examine the separate and combined impact of host MHC (H-2a, H-2ab, H-2b), host gender (two sexes), and parasite clone (“AS” and “CW”; Beale et al. 1978). Variation in age was minimized by synchronized breeding. When age was included as a covariate in the statistical models, the results did not change qualitatively and significant P-values tended to drop slightly (results not shown). Body weights and blood cell densities around exposure (day 0 and 1, respectively) were not significantly different among the experimental groups, except that, as expected (Suckow et al. 2000), males were initially heavier than females (t = 11.0, P < 0.0001) and had lower blood cell densities (t = 3.0, P = 0.004). We tracked the time course of the disease through daily weight measurements and in repeated parasitemia counts and blood cell counts. The resulting repeated-measures analyses of variances (ANOVAs) are summarized in Table 1 and discussed below. We then used these findings to specifically compare the performance of MHC heterozygotes to the average performance of the homozygotes.
TABLE 1 .
The effect of host MHC, parasite clone, and host gender on the time course of disease symptoms
|
Disease symptom |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parasitemia |
Blood cell counts |
Body weight change |
|||||||
|
F |
d.f. |
P |
F |
d.f. |
P |
F |
d.f. |
P |
|
| Between subjects | |||||||||
| Host MHC | 9.6 | 2, 94 | 0.0002 | 5.2 | 2, 94 | 0.007 | 1.2 | 2, 94 | 0.29 |
| Plasmodium clone | 5.9 | 1, 94 | 0.017 | 0.1 | 1, 94 | 0.79 | 3.4 | 1, 94 | 0.07 |
| Host gender | 0.02 | 1, 94 | 0.89 | 27.8 | 1, 94 | <0.0001 | 0.1 | 1, 94 | 0.77 |
| MHC × clone | 0.8 | 2, 94 | 0.43 | 0.5 | 2, 94 | 0.61 | 0.3 | 2, 94 | 0.75 |
| MHC × gender | 0.9 | 2, 94 | 0.39 | 1.2 | 2, 94 | 0.32 | 0.2 | 2, 94 | 0.82 |
| Clone × gender | 10.1 | 1, 94 | 0.002 | 9.5 | 1, 94 | 0.003 | 0.4 | 1, 94 | 0.52 |
| MHC × clone × gender | 0.7 | 2, 94 | 0.49 | 1.2 | 2, 94 | 0.31 | 1.2 | 2, 94 | 0.31 |
| Within subjects (repeated measurements on individual mice) | |||||||||
| Time | 296.7 | 5, 90 | <0.0001 | 166.1 | 10, 85 | <0.0001 | 33.1 | 18, 77 | <0.0001 |
| Time × MHC | 2.4 | 10, 180 | 0.01 | 1.2 | 20, 170 | 0.26 | 1.0 | 36, 154 | 0.46 |
| Time × clone | 6.7 | 5, 90 | <0.0001 | 2.9 | 10, 85 | 0.003 | 1.7 | 18, 77 | 0.05 |
| Time × gender | 1.5 | 5, 90 | 0.20 | 1.9 | 10, 85 | 0.06 | 6.2 | 18, 77 | <0.0001 |
| Time × MHC × clone | 0.4 | 10, 180 | 0.92 | 0.6 | 20, 170 | 0.94 | 1.2 | 36, 154 | 0.27 |
| Time × MHC × gender | 0.7 | 10, 180 | 0.72 | 1.0 | 20, 170 | 0.46 | 0.8 | 36, 154 | 0.78 |
| Time × clone × gender | 1.1 | 5, 90 | 0.38 | 1.7 | 10, 85 | 0.10 | 1.9 | 18, 77 | 0.03 |
| Time × MHC × clone × gender |
1.4 |
10, 180 |
0.18 |
0.7 |
20, 170 |
0.86 |
0.8 |
36, 154 |
0.72 |
The experiment was designed for a fully factorial repeated-measures analysis of variance (ANOVA), incorporating the fixed-effect factors “host MHC,” “host gender,” and “Plasmodium clone” with repeated measures of the following dependent variables: parasitemia (6 measurements from day 4 to day 14), blood cell counts (11 measurements from day 1 to day 22), and body weight (19 measurements from day 4 to day 22), given as differences from the weight at day 0. For within-subject analyses, we used the multivariate F-tests or Wilk's λ (when a factor had more than two levels as in “MHC”).
Consistent with previous studies (Taylor et al. 1998; Mackinnon and Read 1999), mean parasitemia rose dramatically during the first 10 days postinfection (p.i.), and minimal blood cell counts and body weights on average were reached at day 10 and 11 p.i., respectively. At that stage, the mice on average had lost 2.2% (±0.7 SE) of their initial body weight and 58.1% (±2.3) of their initial red blood cells. All mice survived the acute phase of the infection and recovered as parasitemia declined over the next few days.
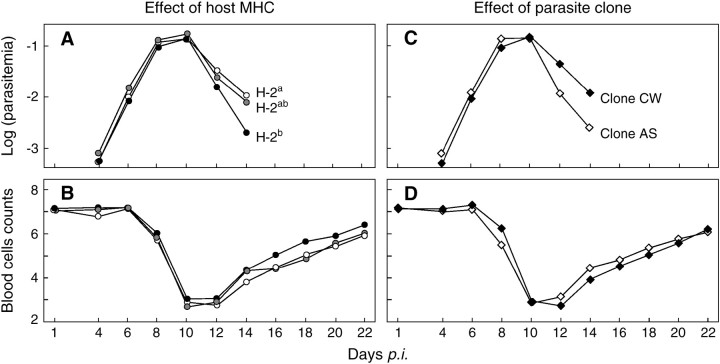
The host's MHC genotype had a strong effect on parasitemia and on blood cell counts, but did not significantly affect body weight change (Table 1). The homozygous H-2a appeared to be more susceptible than the homozygous H-2b type, confirming previous findings comparing inbred lines but where background genetics and maternal effects were not fully controlled (Wunderlich et al. 1988). MHC genotype also influenced the time course of parasitemia (time × MHC in Table 1): the homozygous H-2b type seemed to clear its parasites faster than H-2ab, followed by H-2a (Figure 1A). The response of the heterozygous H-2ab was between the two homozygous genotypes (Figure 1, A and B; see below).
Figure 1.—
The effects of MHC and parasite clone on the course of disease symptoms. The mean parasitemia (log10 transformed) and the mean blood cell number (×109)/ml blood for MHC genotypes H-2a (open circles), H-2b (solid circles), and H-2ab (shaded circles), or clone AS (open diamonds) and clone CW (solid diamonds) are given. See supplementary material at http://www.genetics.org/supplemental/ for details about the experimental procedure and Table 1 for statistics.
We found differences between the parasite clones that are consistent with previous studies (Mackinnon and Read 1999). Clone CW reached overall higher parasitemia (Table 1), but clone AS had its peak parasitemia earlier than clone CW (time × clone in Table 1; Figure 1C). This corresponds to a similar pattern in the time course of the blood cell counts (time × clone in Table 1; Figure 1D). The two clones caused different disease patterns not only because of intrinsic clone-specific characteristics but also depending on host characteristics: the two sexes react differently to the parasite clones (significant clone × gender interaction, Table 1). Gender-specific virulence is common in vertebrates (Zuk and McKean 1996), but here we show an interaction between gender and parasite clone. Specifically, clone CW reached higher parasitemia than clone AS only in female hosts, while in male hosts, the two clones differed in the time course of their parasitemia, with clone AS reaching its peak parasitemia earlier but also disappearing earlier than clone CW (data not shown). However, there was no significant interaction between parasite clone and MHC genotype in any of the traits (Table 1). This suggests that the two clones are similar in at least some of their MHC-presented peptides, such that, for a given parasite clone, virulence is relatively stable across host MHC types.
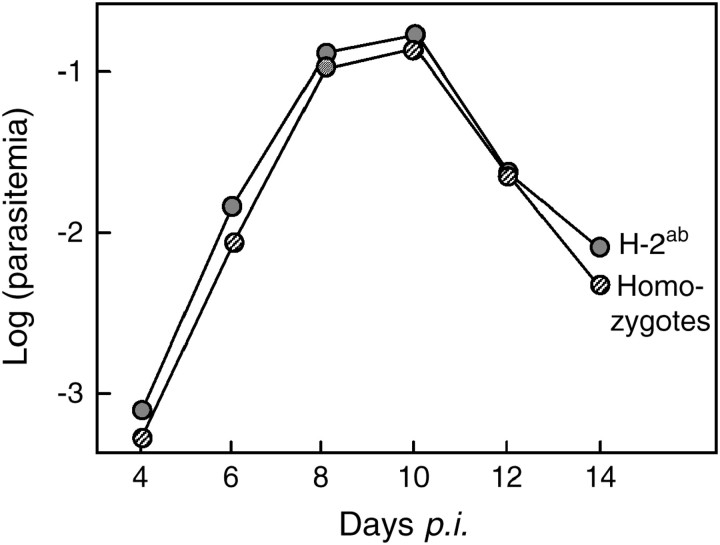
The MHC heterozygotes in our study were neither superior nor inferior to the respective homozygotes; i.e., we found no evidence for overdominance of resistance or susceptibilty (Figure 1). Nor were heterozygotes more resistant than what would be expected from the average of the two homozygotes; i.e., the apparent resistance of the H-2b genotype was not dominant. Indeed, contrary to what is commonly assumed (see references above), the MHC heterozygotes in our study reached significantly higher parasitemias than the average of the two homozygotes (Figure 2). This pattern cannot be explained by differences in starting conditions, as all genotypes were similar in age, weight, gender, and parasite clone (P always > 0.25). A number of factors may explain cases of poor heterozygote performance, including that MHC heterozygosity has an impact on T-cell receptor repertoire selection (Robey and Fowlkes 1994; Vukusic et al. 1995) or that parasite-generated T-cell antagonism, which is known to reduce the efficacy of T-cell-mediated immunity in malaria (Gilbert et al. 1998), has an even more pronounced effect in heterozygotes than in homozygotes. Whatever the physiological explanation, we can conclude that the widely assumed and sometimes supported MHC heterozygosity advantage is only a rule with exceptions. It remains, however, unclear whether this conclusion is true for both class I and class II genes (the congenic lines that we used vary over the entire MHC region). Differences in the recognition system or in gene dose effects between class I and class II genes (Dorf et al. 1979; Moore et al. 1980) could potentially influence antigen recognition under homozygous or heterozygous conditions.
Figure 2.—
The effect of the H-2ab genotype vs. the average effects of the respective homozygote MHC types during infection with P. chabaudi. To test whether the heterozygotes (shaded circles) were as susceptible as the average of the two homozygotes (striped circles), we first equalized the sample size of the homozygous variants before pooling them. We did that by randomly reducing the larger group to the sample size of the smaller group. We then calculated the repeated-measures ANOVAs (fixed factors: heterozygosity, gender, and parasite clone) with the reduced sample size. The average of 10 randomly reduced samples is given. The heterozygosity effect in the repeated-measures ANOVAs (average of 10 runs ± SE) is F1,76 = 6.0 ± 0.62, P = 0.026 ± 0.007. The heterozygosity effect averaged over all days was also significant when tested with the method of linear contrasts on the nonreduced sample size (i.e., using weights of 0.5 for each of the homozygotes and −1 for the heterozygotes and using the between-mouse variance as the residual: t93 = 2.63, P = 0.01).
The link between MHC and human malaria (Hill et al. 1991, 1992) is an often cited example of the influence of MHC genes on the course of a disease. However, for humans, such links cannot be studied under controlled experimental conditions. Other studies used inbred mouse strains that were congenic with respect to the MHC (Wunderlich et al. 1988; Bagot et al. 2002; Cigel et al. 2003), but they did not control for a number of potentially confounding effects. For example, mother's age is known to affect offspring size, number, and general vigor (Finn 1963; Tarin et al. 2004). Maternal effects could explain why some congenic strains produce different olfactory signals in parental strains but not in F2 segregants (Carroll et al. 2002) or why congenic strains sometimes differ in behavior (Heimrich et al. 1988). In addition, MHC-congenic lines may differ with respect to the mutation load on their background genes, as there is at least one example of different mortalities during early development (Wedekind et al. 1996). Variation in maternal effects or mutation load could interact with pathogen susceptibilities (Carroll and Potts 2001). Thus, for studies that aim to isolate the effects of particular loci, it is critical to use breeding designs that randomize all background effects (Carroll and Potts 2001; Wolfer et al. 2002; Wedekind et al. 2004).
In conclusion, we found that, when tested under rigorous experimental conditions, variation in the MHC can have a significant effect on the course of Plasmodium infection, but that MHC heterozygote advantage through overdominance or dominance of resistance cannot be assumed. It remains unclear whether and how our finding is related to the fact that Plasmodium is a comparatively large organism with a presumably large antigen repertoire. However, recent studies on pathogens with a presumably smaller antigen repertoire (Theiler's virus and Salmonella) confirm that MHC heterozygote advantage cannot generally be assumed in the case of single-clone infections (Penn et al. 2002; McClelland et al. 2003). Future studies on malaria might incorporate a wider range of parasite clones and host H2 genotypes or might focus on the effects of multiple-clone infections where the diversity of parasite antigens confronting the MHC may change this result. In the case of Plasmodium, genetically variable infections are harder to clear and are sometimes more virulent than single-clone infections (Taylor et al. 1998; De Roode et al. 2003) and thus may lead to different host-parasite interactions.
Acknowledgments
We thank L. Stevenson and J. Verth for assistance with mouse breeding, H. Hofer, M. Güntert, D. Penn, A. Read, and T. Rülicke, for discussion and/or support, and N. Takahata and two reviewers for comments on the manuscript. C.W. and M.W. are supported by the Swiss National Science Foundation, and C.W. by a Sarah and Daniel Hrdy visiting fellowship.
Footnotes
Communicating editor: N. Takahata
References
- Apanius, V., D. Penn, P. R. Slev, L. R. Ruff and W. K. Potts, 1997. The nature of selection on the major histocompatibility complex. Crit. Rev. Immunol. 17: 179–224. [DOI] [PubMed] [Google Scholar]
- Bagot, S., M. I. Boubou, S. Campino, C. Behrschmidt, O. Gorgette et al., 2002. Susceptibility to experimental cerebral malaria induced by Plasmodium berghei ANKA in inbred mouse strains recently derived from wild stock. Infect. Immun. 70: 2049–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale, G. H., R. Carter and D. Walliker, 1978 Genetics, pp. 213–245 in Rodent Malaria, edited by R. Killick-Kendrick and W. Peters. Academic Press, London.
- Black, F. L., and P. W. Hedrick, 1997. Strong balancing selection at HLA loci: evidence from segregation in South Amerindian families. Proc. Natl. Acad. Sci. USA 94: 12452–12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington, M., G. W. Nelson, M. P. Martin, T. Kissner, D. Vlahov et al., 1999. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 283: 1748–1752. [DOI] [PubMed] [Google Scholar]
- Carroll, L. S., and W. K. Potts, 2001. Accumulated background variation among H2 mutant congenic strains: elimination through PCR-based genotyping of F-2 segregants. J. Immunol. Methods 257: 137–143. [DOI] [PubMed] [Google Scholar]
- Carroll, L. S., D. J. Penn and W. K. Potts, 2002. Discrimination of MHC-derived odors by untrained mice is consistent with divergence in peptide-binding region residues. Proc. Natl. Acad. Sci. USA 99: 2187–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigel, F., J. Batchelder, J. M. Burns, D. Yanez, H. van der Heyde et al., 2003. Immunity to blood-stage murine malarial parasites is MHC class II dependent. Immunol. Lett. 89: 243–249. [DOI] [PubMed] [Google Scholar]
- De Roode, J. C., A. F. Read, B. H. K. Chan and M. J. Mackinnon, 2003. Rodent malaria parasites suffer from the presence of conspecific clones in three-clone Plasmodium chabaudi infections. Parasitology 127: 411–418. [DOI] [PubMed] [Google Scholar]
- Doherty, P. C., and R. M. Zinkernagel, 1975. Enhanced immunological surveillance in mice heterozygous at H-2 gene complex. Nature 256: 50–52. [DOI] [PubMed] [Google Scholar]
- Dorf, M. E., J. H. Stimpfling and B. Benacerraf, 1979. Gene dose effects in Ir gene-controlled systems. J. Immunol. 123: 269–271. [PubMed] [Google Scholar]
- Finn, C. A., 1963. Reproductive capacity and litter size in mice: effect of age and environment. J. Reprod. Fertil. 6: 205. [DOI] [PubMed] [Google Scholar]
- Gilbert, S. C., M. Plebanski, S. Gupta, J. Morris, M. J. Cox et al., 1998. Association of malaria parasite population structure, HLA, and immunological antagonism. Science 279: 1173–1177. [DOI] [PubMed] [Google Scholar]
- Hedrick, P. W., and G. Thomson, 1983. Evidence for balancing selection at Hla. Genetics 104: 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimrich, B., H. Schwegler, W. E. Crusio and W. Buselmaier, 1988. Substrain divergence in C3h inbred mice. Behav. Genet. 18: 671–674. [DOI] [PubMed] [Google Scholar]
- Hill, A. V. S., C. E. M. Allsopp, D. Kwiatkowski, N. M. Anstey, P. Twumasi et al., 1991. Common West African HLA antigens are associated with protection from severe malaria. Nature 352: 595–600. [DOI] [PubMed] [Google Scholar]
- Hill, A. V. S., J. Elvin, A. C. Willis, M. Aidoo, C. E. M. Allsopp et al., 1992. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature 360: 434–439. [DOI] [PubMed] [Google Scholar]
- Lipsitch, M., C. T. Bergstrom and R. Antia, 2003. Effect of human leukocyte antigen heterozygosity on infectious disease outcome: the need for allele-specific measures. BMC Med. Genet. 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon, M. J., and A. F. Read, 1999. Genetic relationships between parasite virulence and transmission in the rodent malaria Plasmodium chabaudi. Evolution 53: 689–703. [DOI] [PubMed] [Google Scholar]
- McClelland, E. E., D. J. Penn and W. K. Potts, 2003. Major histocompatibility complex heterozygote superiority during coinfection. Infect. Immun. 71: 2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, M. J., D. E. Singer and R. M. Williams, 1980. Linkage of severity of experimental allergic encephalomyelitis to the rat major histocompatibility locus. J. Immunol. 124: 1815–1820. [PubMed] [Google Scholar]
- Penn, D. J., K. Damjanovich and W. K. Potts, 2002. MHC heterozygosity confers a selective advantage against multiple-strain infections. Proc. Natl. Acad. Sci. USA 99: 11260–11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey, E., and B. J. Fowlkes, 1994. Selective events in T-cell development. Annu. Rev. Immunol. 12: 675–705. [DOI] [PubMed] [Google Scholar]
- Suckow, M. A., P. Danneman and C. Brayton, 2000 The Laboratory Mouse. CRC Press, London.
- Tarin, J. J., V. Gomez-Piquer, F. Rausell, C. Hermenegildo and A. Cano, 2004. Effect of delayed breeding on the reproductive performance of female mice. Reprod. Fertil. Dev. 16: 373–378. [DOI] [PubMed] [Google Scholar]
- Taylor, L. H., M. J. Mackinnon and A. F. Read, 1998. Virulence of mixed-clone and single-clone infections of the rodent malaria Plasmodium chabaudi. Evolution 52: 583–591. [DOI] [PubMed] [Google Scholar]
- Thursz, M. R., H. C. Thomas, B. M. Greenwood and A. V. S. Hill, 1997. Heterozygote advantage for HLA class-II type in hepatitis B virus infection. Nat. Genet. 17: 11–12. [DOI] [PubMed] [Google Scholar]
- Vukusic, B., L. Poplonski, L. Phillips, J. Pawling, T. Delovitch et al., 1995. Both MHC and background gene heterozygosity alter T cell receptor repertoire selection in an antigen-specific response. Mol. Immunol. 32: 1355–1367. [DOI] [PubMed] [Google Scholar]
- Wedekind, C., M. Chapuisat, E. Macas and T. Rülicke, 1996. Non-random fertilization in mice correlates with the MHC and something else. Heredity 77: 400–409. [DOI] [PubMed] [Google Scholar]
- Wedekind, C., M. Walker, J. Portmann, B. Cenni, R. Müller et al., 2004. MHC-linked susceptibility to a bacterial infection, but no MHC-linked cryptic female choice in whitefish. J. Evol. Biol. 17: 11–18. [DOI] [PubMed] [Google Scholar]
- Wolfer, D. P., W. E. Crusio and H. P. Lipp, 2002. Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends Neurosci. 25: 336–340. [DOI] [PubMed] [Google Scholar]
- Wunderlich, F., H. Mossmann, M. Helwig and G. Schillinger, 1988. Resistance to Plasmodium chabaudi in B-10 mice: influence of the H-2-complex and testosterone. Infect. Immun. 56: 2400–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk, M., and K. A. McKean, 1996. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 26: 1009–1024. [PubMed] [Google Scholar]