Abstract
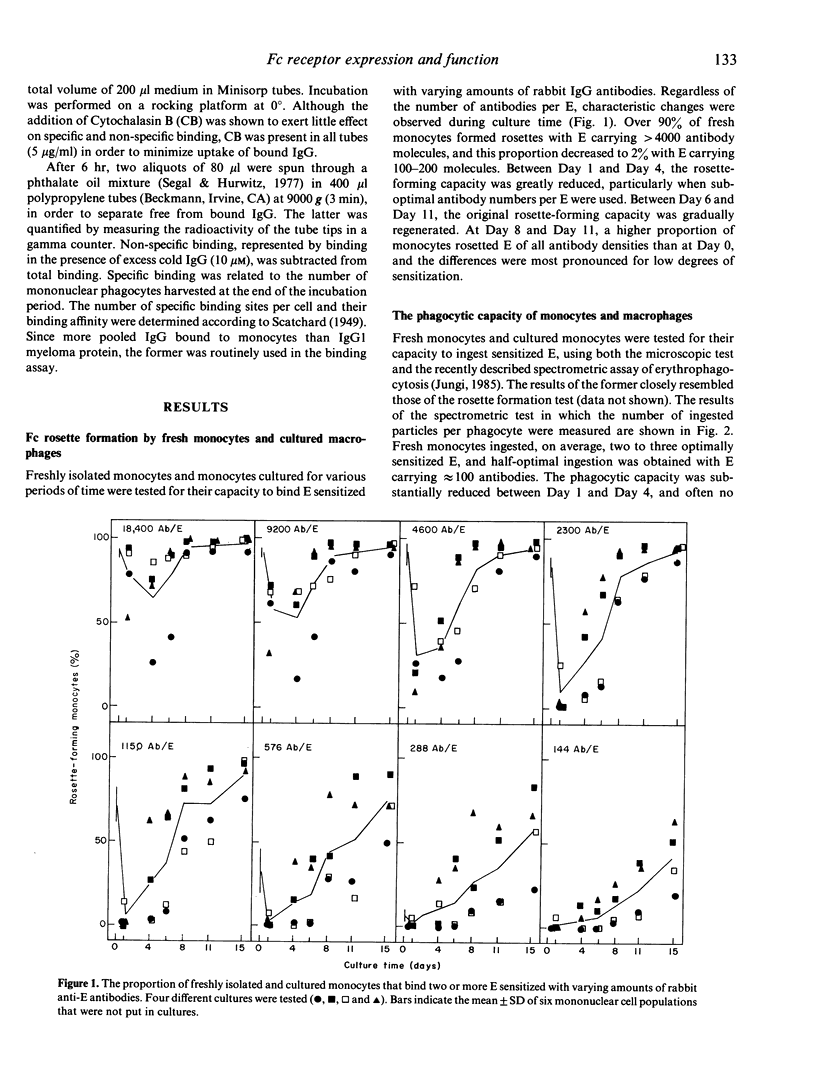
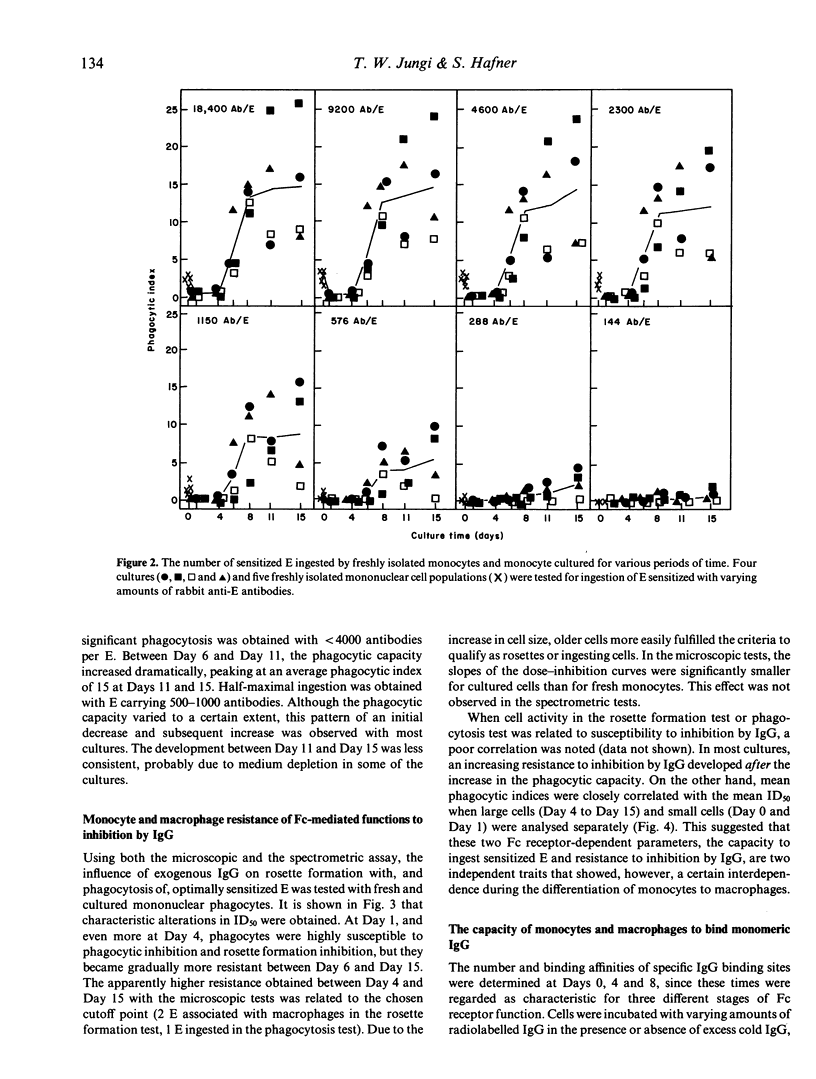
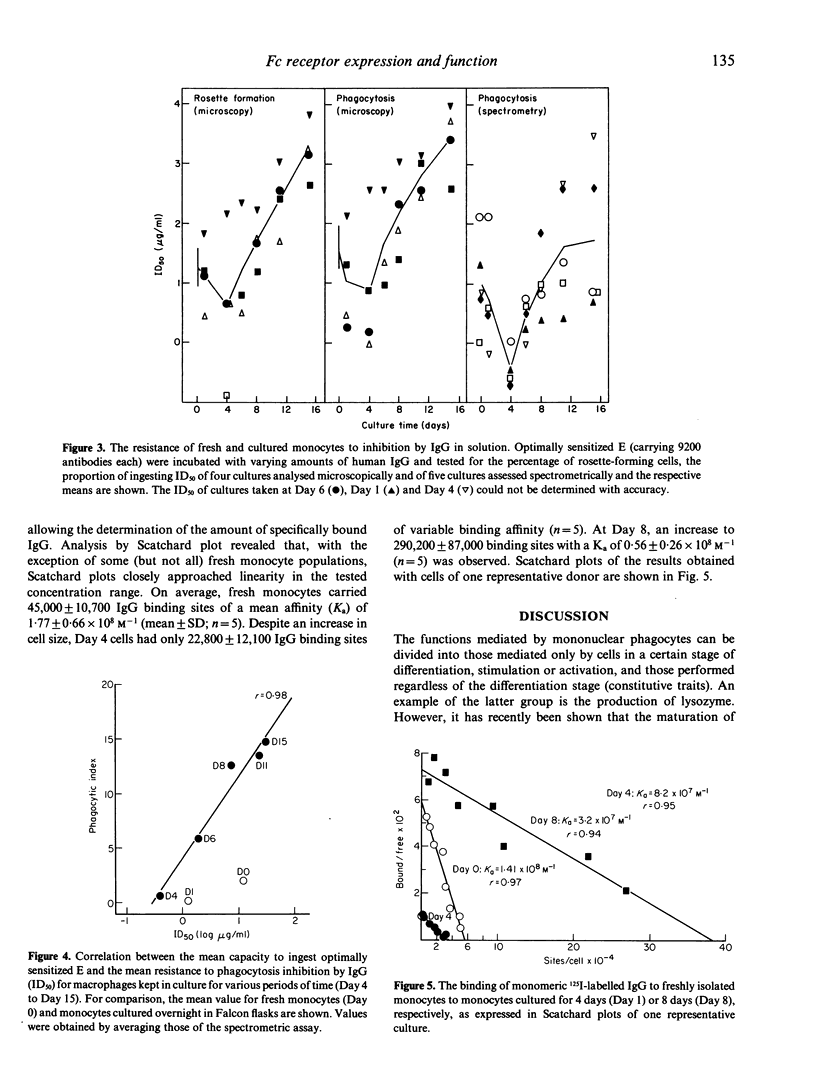
The expression and function of IgG Fc receptors on peripheral blood monocytes and monocytes differentiating in vitro, within hydrophobic membranes, to macrophages have been determined. Three characteristic functional stages could be discerned, and these were reflected in the surface expression of specific IgG binding sites. Stage 1, represented by fresh monocytes, is characterized by efficient binding and phagocytosis of IgG-coated particles, although uptake was limited by the cell size. Within 1 day of culture, monocytes transform into Stage 2 cells. These bind and ingest particles with high IgG surface density only and these functions are exquisitely sensitive to inhibition by IgG in solution. The functional loss is paralleled by a decrease of the number of specific IgG binding sites, despite a gradual increase in cell size. Between Day 4 and Day 8, macrophages acquired the capacity to bind and ingest particles opsonized to a low degree, their phagocytic capacity is strongly enhanced, the number of IgG binding sites increases by a factor greater than 10, despite a moderate increase in cell size only. Mature human macrophages have about six times the Fc receptor number of blood monocytes, but the average binding affinity is decreased. It appears that the phagocytic capacity is related to the number of IgG binding sites, and the susceptibility to inhibition by IgG in solution is related to their surface density.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. D., Andrews J. A., Leslie R. G., Wood N. J. The binding of human and guinea-pig IgG subclasses to homologous macrophage and monocyte Fc receptors. Immunology. 1978 Jul;35(1):115–123. [PMC free article] [PubMed] [Google Scholar]
- Anderson C. L., Abraham G. N. Characterization of the Fc receptor for IgG on a human macrophage cell line, U937. J Immunol. 1980 Dec;125(6):2735–2741. [PubMed] [Google Scholar]
- Andreesen R., Picht J., Löhr G. W. Primary cultures of human blood-born macrophages grown on hydrophobic teflon membranes. J Immunol Methods. 1983 Feb 11;56(3):295–304. doi: 10.1016/s0022-1759(83)80019-2. [DOI] [PubMed] [Google Scholar]
- Carter S. D., Leslie R. G., Reeves W. G. Human monocyte binding of homologous monomer and complexed IgG. Immunology. 1982 Aug;46(4):793–800. [PMC free article] [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries L. F., Hall R. P., Lawley T. J., Crabtree G. R., Frank M. M. Monocyte receptors for the Fc portion of IgG studies with monomeric human IgG1: normal in vitro expression of Fc gamma receptors in HLA-B8/Drw3 subjects with defective Fc gamma-mediated in vivo clearance. J Immunol. 1982 Sep;129(3):1041–1049. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Jungi T. W. A rapid and sensitive method allowing photometric determination of erythrophagocytosis by mononuclear phagocytes. J Immunol Methods. 1985 Sep 3;82(1):141–153. doi: 10.1016/0022-1759(85)90233-9. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., Barandun S. Estimation of the degree of opsonization of homologous erythrocytes by IgG for intravenous and intramuscular use. Vox Sang. 1985;49(1):9–19. doi: 10.1111/j.1423-0410.1985.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Gaudernack G. In vitro differentiation of human monocytes. Differences in monocyte phenotypes induced by cultivation on glass or on collagen. J Exp Med. 1982 Oct 1;156(4):1101–1114. doi: 10.1084/jem.156.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurlander R. J., Batker J. The binding of human immunoglobulin G1 monomer and small, covalently cross-linked polymers of immunoglobulin G1 to human peripheral blood monocytes and polymorphonuclear leukocytes. J Clin Invest. 1982 Jan;69(1):1–8. doi: 10.1172/JCI110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurlander R. J., Haney A. F., Gartrell J. Human peritoneal macrophages possess two populations of IgG Fc receptors. Cell Immunol. 1984 Jul;86(2):479–490. doi: 10.1016/0008-8749(84)90403-9. [DOI] [PubMed] [Google Scholar]
- Kurlander R. J. Reversible and irreversible loss of Fc receptor function of human monocytes as a consequence of interaction with immunoglobulin G. J Clin Invest. 1980 Oct;66(4):773–781. doi: 10.1172/JCI109915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Unkeless J. C., Pieczonka M. M., Silverstein S. C. Modulation of Fc receptors of mononuclear phagocytes by immobilized antigen-antibody complexes. Quantitative analysis of the relationship between ligand number and Fc receptor response. J Exp Med. 1983 Jun 1;157(6):1746–1757. doi: 10.1084/jem.157.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983 Jan 28;56(2):261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- Newman S. L., Musson R. A., Henson P. M. Development of functional complement receptors during in vitro maturation of human monocytes into macrophages. J Immunol. 1980 Nov;125(5):2236–2244. [PubMed] [Google Scholar]
- Perussia B., Dayton E. T., Lazarus R., Fanning V., Trinchieri G. Immune interferon induces the receptor for monomeric IgG1 on human monocytic and myeloid cells. J Exp Med. 1983 Oct 1;158(4):1092–1113. doi: 10.1084/jem.158.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier C. G., Inada S., Fries L. F., Takahashi T., Frank M. M., Brown E. J. Plasma fibronectin enhances phagocytosis of opsonized particles by human peripheral blood monocytes. J Exp Med. 1983 Jun 1;157(6):1844–1854. doi: 10.1084/jem.157.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D. M., Hurwitz E. Binding of affinity cross-linked oligomers of IgG to cells bearing Fc receptors. J Immunol. 1977 Apr;118(4):1338–1337. [PubMed] [Google Scholar]
- Treves A. J., Yagoda D., Haimovitz A., Ramu N., Rachmilewitz D., Fuks Z. The isolation and purification of human peripheral blood monocytes in cell suspension. J Immunol Methods. 1980;39(1-2):71–80. doi: 10.1016/0022-1759(80)90295-1. [DOI] [PubMed] [Google Scholar]
- Walker W. S. Separate Fc-receptors for immunoglogulins IgG2a and IgG2b on an established cell line of mouse macrophages. J Immunol. 1976 Apr;116(4):911–914. [PubMed] [Google Scholar]
- Wright S. D., Craigmyle L. S., Silverstein S. C. Fibronectin and serum amyloid P component stimulate C3b- and C3bi-mediated phagocytosis in cultured human monocytes. J Exp Med. 1983 Oct 1;158(4):1338–1343. doi: 10.1084/jem.158.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuest D., Crane R., Rinehart J. J. Enhancement of Fc receptor function during human monocyte differentiation in vitro. J Reticuloendothel Soc. 1981 Aug;30(2):147–155. [PubMed] [Google Scholar]
- van der Meer J. W., Bulterman D., van Zwet T. L., Elzenga-Claasen I., van Furth R. Culture of mononuclear phagocytes on a teflon surface to prevent adherence. J Exp Med. 1978 Jan 1;147(1):271–276. doi: 10.1084/jem.147.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]


