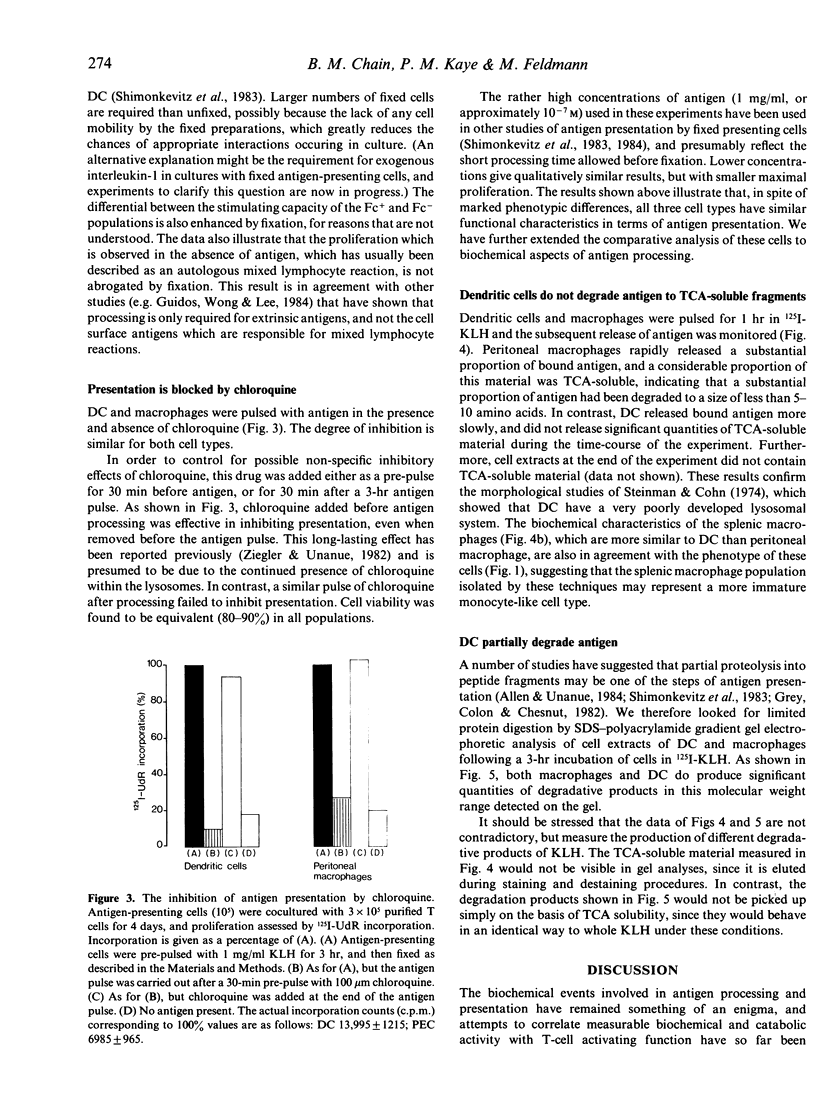
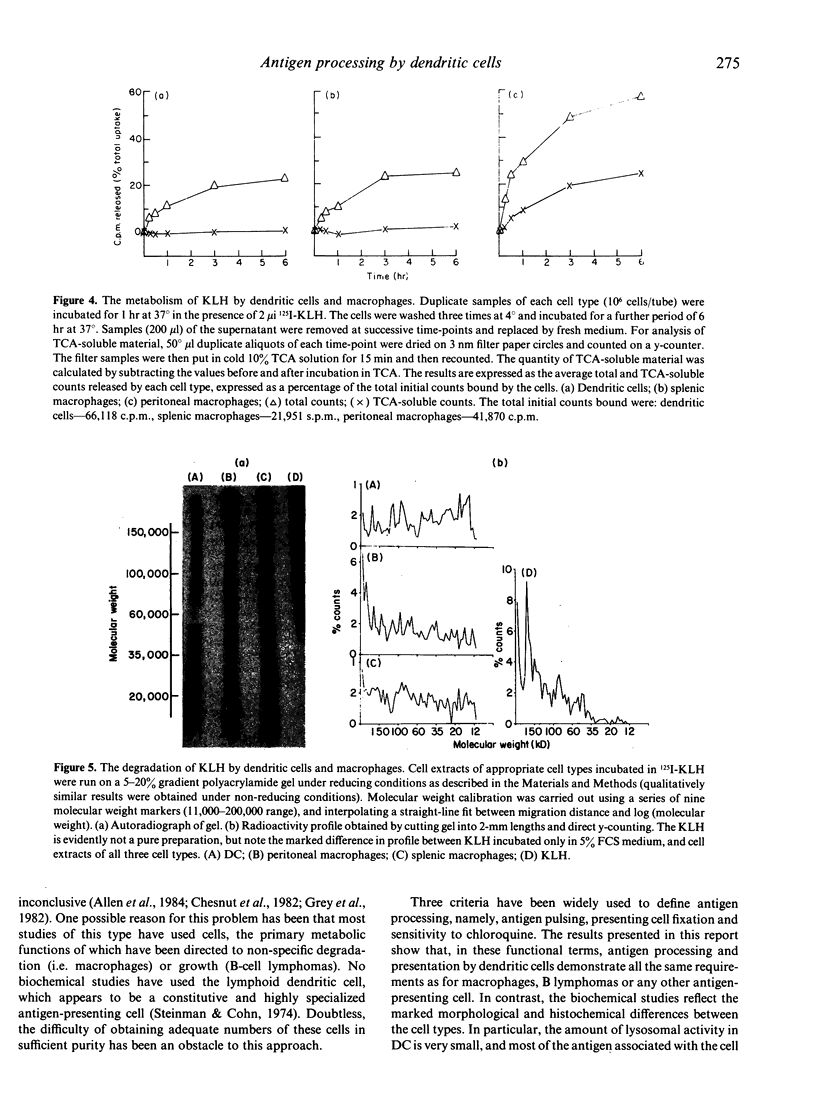
Abstract
The response of primed T cells to keyhole limpet haemocyanin (KLH) was used to compare the characteristics of antigen presentation by lymphoid dendritic cells, splenic and peritoneal macrophages. In a similar manner to macrophages, purified dendritic cells could be pulsed with antigen and subsequently fixed by brief glutaraldehyde fixation and still retain antigen presenting activity. Also, as previously reported for macrophages, presentation could be inhibited by chloroquine. These functional experiments suggested that the pathway of antigen presentation in dendritic cells and macrophages was similar or identical. However, biochemical studies, using radiolabelled antigen, showed that dendritic cells do not significantly degrade large proteins such as KLH to TCA-soluble form, but partially hydrolyse them to smaller peptide fragments. The significance of these results in terms of a model of the cellular pathways of antigen presentation is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Beller D. I., Braun J., Unanue E. R. The handling of Listeria monocytogenes by macrophages: the search for an immunogenic molecule in antigen presentation. J Immunol. 1984 Jan;132(1):323–331. [PubMed] [Google Scholar]
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Banda M. J., Werb Z. Mouse macrophage elastase. Purification and characterization as a metalloproteinase. Biochem J. 1981 Feb 1;193(2):589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut R. W., Colon S. M., Grey H. M. Antigen presentation by normal B cells, B cell tumors, and macrophages: functional and biochemical comparison. J Immunol. 1982 Apr;128(4):1764–1768. [PubMed] [Google Scholar]
- Fraser J. D., Scott G. K. Mitogenic proteinases from human leukocytes. Mol Immunol. 1984 Apr;21(4):311–320. doi: 10.1016/0161-5890(84)90102-0. [DOI] [PubMed] [Google Scholar]
- Golstein P., Blomgren H. Further evidence for autonomy of T cells mediating specific in vitro cytotoxicity: efficiency of very small amounts of highly purified T cells. Cell Immunol. 1973 Oct;9(1):127–141. doi: 10.1016/0008-8749(73)90174-3. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Colon S. M., Chesnut R. W. Requirements for the processing of antigen by antigen-presenting B cells. II. Biochemical comparison of the fate of antigen in B cell tumors and macrophages. J Immunol. 1982 Dec;129(6):2389–2395. [PubMed] [Google Scholar]
- Guidos C., Wong M., Lee K. C. A comparison of the stimulatory activities of lymphoid dendritic cells and macrophages in T proliferative responses to various antigens. J Immunol. 1984 Sep;133(3):1179–1184. [PubMed] [Google Scholar]
- Joiner K. A., Warren K. A., Hammer C., Frank M. M. Bactericidal but not nonbactericidal C5b-9 is associated with distinctive outer membrane proteins in Neisseria gonorrhoeae. J Immunol. 1985 Mar;134(3):1920–1925. [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Hinuma S., Tada M. Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods. 1979;29(1):17–25. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- McKean D. J., Nilson A., Infante A. J., Kazim L. Biochemical characterization of B lymphoma cell antigen processing and presentation to antigen-reactive T cells. J Immunol. 1983 Dec;131(6):2726–2734. [PubMed] [Google Scholar]
- Nowell J., Quaranta V. Chloroquine affects biosynthesis of Ia molecules by inhibiting dissociation of invariant (gamma) chains from alpha-beta dimers in B cells. J Exp Med. 1985 Oct 1;162(4):1371–1376. doi: 10.1084/jem.162.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimonkevitz R., Colon S., Kappler J. W., Marrack P., Grey H. M. Antigen recognition by H-2-restricted T cells. II. A tryptic ovalbumin peptide that substitutes for processed antigen. J Immunol. 1984 Oct;133(4):2067–2074. [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974 Feb 1;139(2):380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Nussenzweig M. C. Dendritic cells: features and functions. Immunol Rev. 1980;53:127–147. doi: 10.1111/j.1600-065x.1980.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D., Lavie G., Franklin E. C. Demonstration of membrane-bound proteolytic activity on the surface of mononuclear leukocytes. J Histochem Cytochem. 1981 Mar;29(3A):451–456. doi: 10.1177/29.3.451. [DOI] [PubMed] [Google Scholar]



