Abstract
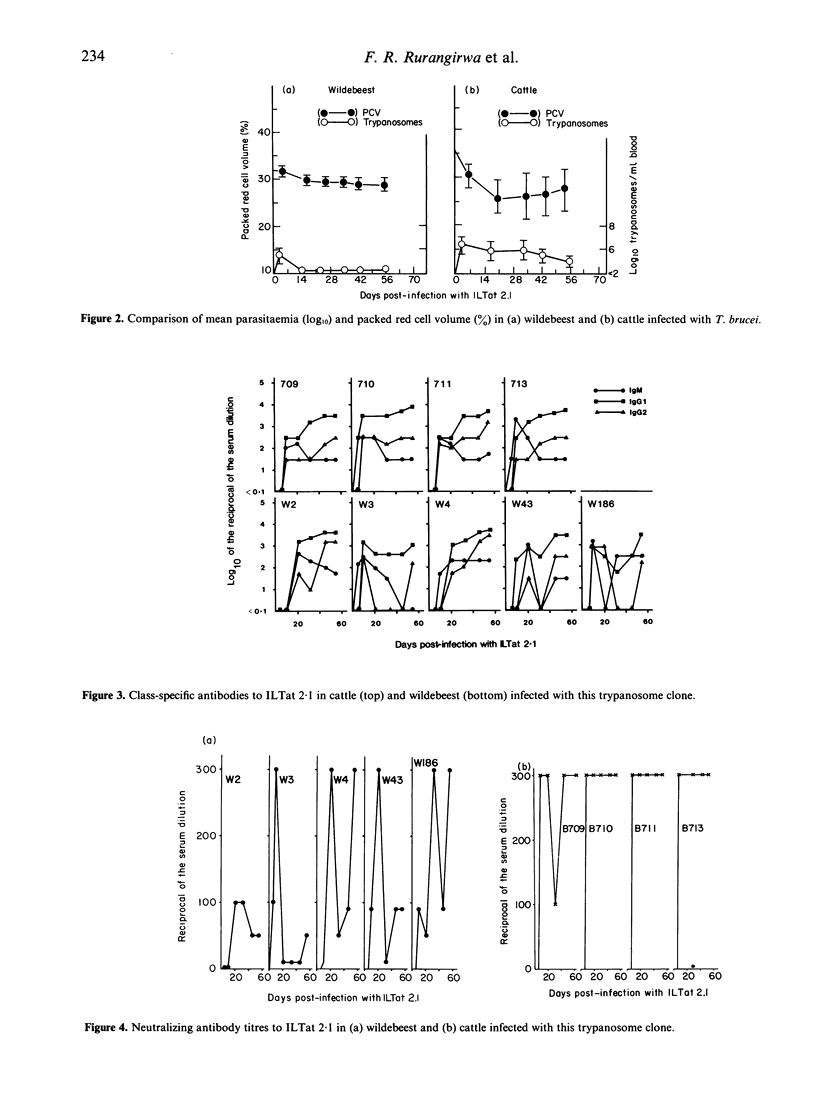
The course of Trypanosoma brucei infection in the wildebeest (Connochaetes taurinus) was studied. A low but persistent parasitaemia developed in all five wildebeest following intravenous inoculation with 1 X 10(8) organisms of clone ILTat 2.1. Unlike cattle controls, however, the wildebeest did not develop anaemia. In both cattle and wildebeest, radioimmunoassay studies revealed a classical sequence of production of IgM, IgG1 and IgG2 antibodies which had the capacity to bind to the corresponding purified variable surface glycoprotein and to neutralize the infectivity of ILTat 2.1. Investigations into the interaction between post-infection sera, trypanosomes and freshly isolated peripheral blood leucocytes (PBL) of wildebeest and cattle showed that sera from the wildebeest had a higher capacity to induce adherence of trypanosomes to homologous PBL. The adherence and phagocytosis-inducing activity resided in the IgM fraction. Cross-testing of the antibodies and PBL revealed that wildebeest IgM antibodies induced high adherence indices when tested on cattle PBL. High adherence indices were also observed when cattle IgM antibodies were tested on PBL of wildebeest. It was concluded that the phagocytic system of the wildebeest was superior to that of cattle, that freshly prepared wildebeest PBL bear receptors for wildebeest as well as cattle IgM, and that cattle PBL bear a receptor for wildebeest IgM that would appear to be different from that for cattle IgM.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalund O., Hoerlein A. B., Adler H. C. The migration test on circulating bovine leukocytes and its possible application in the diagnosis of Johne's disease. Acta Vet Scand. 1970;11(2):331–334. doi: 10.1186/BF03547996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Antigenic variation in trypanosomes. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):240–244. doi: 10.4269/ajtmh.1977.26.240. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975 Dec;71(3):393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- Dillmann J. S., Townsend A. J. A trypanosomiasis survey of wild animals in the Luangwa Valley, Zambia. Acta Trop. 1979 Dec;36(4):349–356. [PubMed] [Google Scholar]
- Fey H., Pfister H., Messerli J., Sturzenegger N., Grolimund F. Methods of isolation, purification and quantitation of bovine immunoglobulins: a technical review. Zentralbl Veterinarmed B. 1976 May;23(4):269–300. doi: 10.1111/j.1439-0450.1976.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Geigy R., Kauffmann M. Sleeping sickness survey in the Serengeti area (Tanzania) 1971. I. Examination of large mammals for trypanosomes. Acta Trop. 1973;30(1):12–23. [PubMed] [Google Scholar]
- Kissling K., Karbe E., Freitas E. K. In vitro phagocytic activity of neutrophils of various cattle breeds with and without Trypanosoma congolense infection. Tropenmed Parasitol. 1982 Sep;33(3):158–160. [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Leid R. W., Williams J. F. The immunological response of the rat to infection with Taenia taeniaeformis. I. Immunoglobulin classes involved in passive transfer of resistance. Immunology. 1974 Aug;27(2):195–208. [PMC free article] [PubMed] [Google Scholar]
- Masake R. A., Musoke A. J., Nantulya V. M. Specific antibody responses to the variable surface glycoproteins of Trypanosoma congolense in infected cattle. Parasite Immunol. 1983 Jul;5(4):345–355. doi: 10.1111/j.1365-3024.1983.tb00750.x. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Musoke A. J., Kurtti T. Functional properties of bovine IgG1 and IgG2: interaction with complement, macrophages, neutrophils and skin. Immunology. 1979 Oct;38(2):249–256. [PMC free article] [PubMed] [Google Scholar]
- Murray M., Clifford D. J., McIntyre W. I. Diagnosis of African trypanosomiasis in the bovine. Trans R Soc Trop Med Hyg. 1979;73(1):120–121. doi: 10.1016/0035-9203(79)90148-2. [DOI] [PubMed] [Google Scholar]
- Nantulya V. M., Musoke A. J., Barbet A. F., Roelants G. E. Evidence for reappearance of Trypanosoma brucei variable antigen types in relapse populations. J Parasitol. 1979 Oct;65(5):673–679. [PubMed] [Google Scholar]
- Ngaira J. M., Nantulya V. M., Musoke A. J., Hirumi K. Phagocytosis of antibody-sensitized Trypanosoma brucei in vitro by bovine peripheral blood monocytes. Immunology. 1983 Jun;49(2):393–400. [PMC free article] [PubMed] [Google Scholar]
- Pinder M., Libeau G., Hirsch W., Tamboura I., Hauck-Bauer R., Roelants G. E. Anti-trypanosome specific immune responses in bovids of differing susceptibility to African trypanosomiasis. Immunology. 1984 Feb;51(2):247–258. [PMC free article] [PubMed] [Google Scholar]
- Rovis L., Dube D. K. Studies on the biosynthesis of the variant surface glycoproteins of Trypanosoma brucei: sequence of glycosylation. Mol Biochem Parasitol. 1981 Nov;4(1-2):77–93. doi: 10.1016/0166-6851(81)90031-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Young A. S., Kanhai G. K., Stagg D. A. Phagocytosis of Trypanosoma (Nannomonas) congolense by circulating macrophages in the African buffalo (Syncerus caffer). Res Vet Sci. 1975 Jul;19(1):108–110. [PubMed] [Google Scholar]



