Abstract
We found that Borrelia burgdorferi-vaccinated gamma interferon-deficient (IFN-γ0) mice challenged with B. burgdorferi developed prominent chronic destructive osteoarthropathy. When these mice were treated with anti-tumor necrosis factor alpha (TNF-α) antibody, the severity of the destructive osteoarthritis was enhanced and affected the mobility of the animals. In addition, extensive swelling of the hind paws occurred. In contrast, treatment of B. burgdorferi-vaccinated, challenged IFN-γ0 mice with recombinant TNF-α (rTNF-α) inhibited the development of arthritis, including swelling of the hind paws. Moreover, treatment of vaccinated, challenged IFN-γ0 mice with anti-TNF-α inhibited fourfold the production of an antibody that kills B. burgdorferi, while treatment of vaccinated, challenged IFN-γ0 mice with rTNF-α slightly elevated the level of the borreliacidal antibody. These results suggest that the level of TNF-α directly or indirectly regulates the production of borreliacidal antibody and the development of vaccine-induced destructive Lyme osteoarthritis. Studies are in progress to determine the mechanism by which TNF-α-dependent cytokines generate the destructive arthritis.
Arthritis is a leading cause of Lyme disease-associated morbidity in the United States, affecting approximately 60% of individuals infected with the tick-borne spirochete Borrelia burgdorferi (37). Intermittent episodes of arthritis develop several weeks or months after infection and, despite adequate antimicrobial therapy, symptoms persist in 10% of patients with arthritis (17, 24, 36, 37). In severe cases, chronic inflammatory Lyme arthritis can lead to cartilage and bone erosion with permanent joint dysfunction (37). Arthritis has also been detected in humans following vaccination with outer surface protein A (OspA) of B. burgdorferi (32). Moreover, it has been shown that arthritis can develop in B. burgdorferi- or OspA-vaccinated hamsters challenged with the Lyme spirochete (11).
The mechanism by which B. burgdorferi interacts with the host immune system to induce arthritis is not fully understood. The development of Lyme arthritis has been associated with T-helper type 1 (Th1)-associated cytokine production (18, 25, 28, 44). Elevated levels of the Th1-cell-associated cytokine gamma interferon (IFN-γ) have been found in mice developing arthritis after infection with B. burgdorferi (26, 28) and in humans with chronic Lyme arthritis (16, 44). Neutralization of IFN-γ ameliorates the severity of the arthritis (26). However, Brown and Reiner (6) presented compelling evidence that IFN-γ is not absolutely required for the induction of arthritis. When IFN-γ-deficient (IFN-γ0) mice were challenged with B. burgdorferi, they developed severe arthritis. This result suggests that other cytokines or immune regulatory mechanisms are responsible for the induction of Lyme arthritis.
Tumor necrosis factor alpha (TNF-α) is a key cytokine that drives inflammation in rheumatoid arthritis (9, 21) and other arthritides (12, 33, 38, 40). In addition, TNF-α acts synergistically with IFN-γ to expand and amplify the inflammatory response (10, 14, 19, 20). Blocking TNF-α with soluble TNF-α receptor protein or with anti-TNF-α antibody attenuates disease activity (23, 31, 40, 42, 43). In this report, we first show that B. burgdorferi-vaccinated IFN-γ0 mice developed severe osteoarthritis after they were challenged with the Lyme spirochete. Furthermore, treatment of vaccinated IFN-γ0 mice with recombinant TNF-α (rTNF-α) decreased the severity of the osteoarthritis when the mice were challenged with B. burgdorferi, whereas anti-TNF-α treatment increased the severity of the disease.
MATERIALS AND METHODS
Mice.
IFN-γ0 mice (parental stain, C57BL/6-129) were obtained from W. P. Weidanz (University of Wisconsin) with permission from Genentech, Inc. (South San Francisco, Calif.). The mice were bred at the animal facility located at the Wisconsin State Laboratory of Hygiene. Six- to 12-week-old inbred IFN-γ0 mice weighing 20 to 30 g were housed four per cage at an ambient temperature of 21°C. Food and acidified water were provided ad libitum. Experimental protocols were reviewed and approved by the Animal Care and Use Committee of the Medical School, University of Wisconsin.
Organisms.
Low-passage (<10) B. burgdorferi isolates 297 (from human spinal fluid) and C-1-11 (from Ixodes scapularis) were grown at 32°C in modified Barbour-Stoenner-Kelly (BSK) medium (7) until reaching a concentration of approximately 107 spirochetes/ml. Samples (500 μl) were then dispensed into 1.5-ml screw-cap tubes (Sarstedt, Newton, N.C.) containing 500 μl of BSK medium supplemented with 10% glycerol (Sigma Chemical Co., St. Louis, Mo.), and the tubes were sealed and stored at −70°C. When needed, a frozen suspension of spirochetes was thawed, and a sample was used to inoculate 4 ml of fresh BSK medium. Spirochetes were viewed by dark-field microscopy and enumerated by using a Petroff-Hausser counting chamber.
Vaccine preparation.
B. burgdorferi isolate 297 organisms were grown in 1 liter of BSK medium for 6 days, pelleted by centrifugation (10,000 × g, 15°C, 10 min), and washed three times with phosphate-buffered saline (PBS; pH 7.4). The washed pellet was resuspended in 1% formalin, incubated at 32°C for 30 min with periodic mixing, washed three times by centrifugation with PBS (10,000 × g, 10°C, 15 min), and resuspended in PBS. Subsequently, the formalin-inactivated spirochetes were mixed with a sufficient volume of 1% aluminum hydroxide (alum; Reheis, Berkeley Heights, N.J.) to yield 4 × 106 spirochetes/ml.
Vaccination of mice.
It was shown previously (27, 34) that vaccination with whole cells of B. burgdorferi in alum and challenge with the Lyme spirochete can elicit severe destructive arthritis. Whole cells are not recommended as a vaccine for human usage. The ability of whole cells to consistently induce arthritis permits evaluation of the cytokine mechanisms responsible for induction or prevention of the arthritis.
Mice were anesthetized with ether contained in a mouth-and-nose cup and injected subcutaneously in the inguinal region with 0.25 ml (approximately 106 B. burgdorferi cells) of the formalin-inactivated vaccine preparation. The suspension contained approximately 100 μg of borrelial protein. Sham-vaccinated mice were injected with either BSK medium or 1% alum alone.
Administration of rTNF-α and anti-TNF-α.
Lyophilized mouse rTNF-α (10 μg) and purified rat anti-mouse TNF-α monoclonal neutralizing antibody (1 mg/ml) were obtained from R & D Systems (Minneapolis, Minn.) and PharMingen (San Diego, Calif.), respectively. The rTNF-α was resuspended in filter-sterilized (0.2-μm-pore-size filter; Acrodisk; Gelman Sciences, Ann Arbor, Mich.) 0.1% bovine serum albumin to yield a concentration of 10 μg/ml. At 21 days after vaccination, two groups of five mice each were injected in the right hind paw with 50 μl of rTNF-α or anti-TNF-α. Within 1 h after the administration of rTNF-α or anti-TNF-α, mice were challenged subcutaneously with 106 B. burgdorferi organisms. rTNF-α (1 μg) and anti-TNF-α (0.1 mg/ml) were injected daily for 7 days. Selection of the concentrations used was based on dose-response curves.
Infection of mice.
At 21 days after vaccination with B. burgdorferi isolate 297 in alum, mice were anesthetized with ether contained in a mouth-and-nose cup and injected subcutaneously in the right hind paw with 50 μl of BSK medium containing 106 B. burgdorferi isolate C-1-11 organisms. Mice were injected with B. burgdorferi isolate C-1-11 because vaccination with B. burgdorferi isolate 297 does not produce protective antibodies that would prevent B. burgdorferi isolate C-1-11 from inducing arthritis (27). Controls included vaccinated and nonvaccinated mice injected with BSK medium or B. burgdorferi isolate C-1-11. In addition, vaccinated mice were challenged with nonviable B. burgdorferi.
Mouse serum samples.
Serum samples were obtained from vaccinated mice anesthetized with ether contained in a mouth-and-nose cup and bled by intracardiac puncture. The blood was pooled for each group and allowed to clot, and serum was separated by centrifugation at 500 × g, divided into 1.5-ml screw-cap tubes, and frozen at −70°C until used. Equal volumes of thawed serum samples from the same group of mice were pooled before use.
Assessment of arthritis.
Swelling of the hind paws of mice was measured to determine the level of the inflammatory response. Before and during experimentation, age-matched male mice were randomly chosen, and their right hind paws were measured to establish a baseline of paw size. After infection, the hind paws were measured every other day for 20 days with a dial-type vernier caliper (Fisher Scientific, Pittsburgh, Pa.) graduated in 0.1-cm increments. Measurements were obtained by anesthetizing each mouse with ether contained in a mouth-and-nose cup and carefully measuring the width and thickness of each right tibiotarsal joint. The mean group value was obtained by dividing the group sum of the caliper values for each hind paw by the number of hind paws per group. This mean value represented the severity of hind-paw swelling.
Preparation of tissues for histologic examination.
At 21 days after infection, mice were euthanized by CO2 asphyxiation. Their hind legs were amputated at midfemur and fixed in 10% neutral buffered zinc-formalin for 24 h. The legs were then placed in decalcifying solution (Lerner Laboratories, Pittsburgh, Pa.) for 24 h, followed by the addition of fresh decalcifying solution for an additional 24 h. Following decalcification, the hind legs were bisected longitudinally, placed in embedding cassettes (Fisher Scientific), embedded in paraffin, cut into 6-μm sections, fixed on glass slides, and stained with hematoxylin and eosin. Sections were cryptically coded for unbiased histopathologic examination by a certified pathologist (D.M.E.).
Detection of borreliacidal antibody by membrane filtration.
Frozen serum samples were thawed, heat inactivated (56°C, 1 min), filter sterilized with a 0.2-μm-pore-size cellulose acetate filter centrifuge tube (Costar, Corning, N.Y.), and serially twofold diluted (1:2 to 1:8,192) with fresh BSK medium. Samples (100 μl) of each dilution were transferred to 1.5-ml screw-cap tubes, and 100 μl of BSK medium containing 104 B. burgdorferi C-1-11 organisms per ml was added along with 20 μl of sterile guinea pig complement (Sigma). The tubes were gently shaken and incubated for 24 and 48 h at 32°C.
After incubation, 100 μl of each suspension was removed and placed in individual 1.5-ml screw-cap tubes. Subsequently, 100 μl of a propidium iodide solution (1.0 mg/ml; Molecular Probes, Eugene, Oreg.) diluted 1:50 in sterile PBS was added. The suspensions were briefly mixed before being incubated at 56°C for 30 min to permit intercalation of propidium iodide into the spirochetes. One hundred microliters of each sample was then filtered through 0.2-μm-pore-size Nuclepore polycarbonate membrane filters (47-mm diameter; Whatman Nuclepore, Clifton, N.J.) under negative pressure with a single-place sterility test manifold (Millipore Corporation, Bedford, Mass.) attached to a vacuum pump. Membrane filters were washed with approximately 8 ml of sterile double-distilled water, removed from the vacuum apparatus, allowed to dry, and placed on glass microscope slides. Coverslips were placed on the filters before viewing by use of a Laborlux S fluorescence microscope (Leitz, Wetzlar, Germany) with a ×50 oil immersion objective.
The number of spirochetes on each filter was quantitated by viewing approximately 30 fields. The borreliacidal antibody titer was defined as the reciprocal of the dilution preceding the dilution at which the number of spirochetes was equal to that in the control. Generally, individual spirochetes with a few clumps were uniformly distributed throughout the fields on the filters of the control sera.
TNF-α enzyme-linked immunosorbent assay.
Sterile 96-well flat-bottom plates (Nunc Maxisorp P/N) were coated with 100 μl of purified rat anti-murine TNF-α monoclonal antibody (26731 E; PharMingen) diluted in PBS (1:250). After incubation overnight at 4°C, wells were washed three times with PBS containing 0.05% Tween 20 (PBS-T). Wells were then blocked with 200 μl of PBS containing 10% fetal bovine serum (PharMingen). After three washes with PBS-T, wells were inoculated in triplicate with 100 μl of standard or sample and incubated for 2 h at 21°C. Subsequently, wells were washed five times with PBS-T and incubated with 100 μl of biotinylated rat anti-murine TNF-α monoclonal antibody diluted 1:250 in PBS containing 10% fetal bovine serum for 1 h. After seven washes with PBS-T, wells were incubated (30 min, 21°C, dark) with 100 μl of tetramethylbenzidine and 100 μl of hydrogen peroxide. Reactions were stopped by the addition of 50 μl of 1 M H3PO4, and absorbances at 450 nm were immediately determined. Absorbance readings for samples were converted into units of picograms per milliliter through the use of a standard curve obtained from an enzyme-linked immunosorbent assay performed with a TNF-α standard. TNF-α was serially diluted twofold in PBS containing 10% fetal bovine serum from an initial concentration of 500 pg/ml to 15.6 pg/ml.
Statistical analyses.
The mean caliper values among groups were tested by analysis of variance with Minitab statistical analysis software. The alpha level was set at 0.05 before the experiments were started. The standard error of the mean for each group mean caliper value was also calculated.
RESULTS
Development of destructive Lyme arthritis in IFN-γ0 mice vaccinated against challenge with B. burgdorferi.
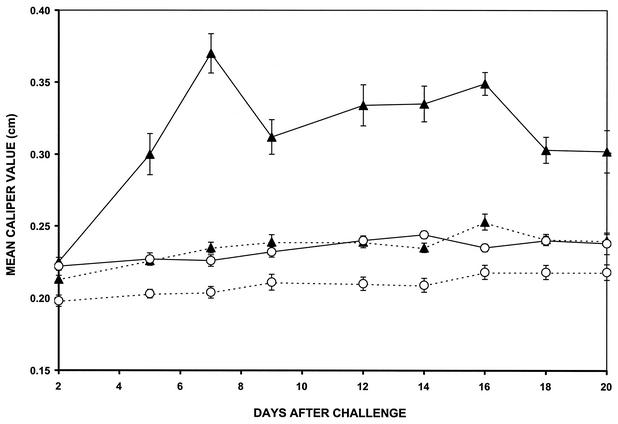
Two groups of five IFN-γ0 mice each were vaccinated with formalin-inactivated B. burgdorferi in alum. At 21 days after vaccination, one group of vaccinated mice was challenged in the hind paws with 106 viable B. burgdorferi organisms. Swelling of the hind paws was detected 5 days after challenge, peaked at day 7, persisted until day 16, and gradually decreased (Fig. 1). No swelling of the hind paws was detected in nonchallenged, vaccinated IFN-γ0 mice. In addition, swelling of the hind paws was not detected in nonvaccinated mice challenged with B. burgdorferi or in nonvaccinated, nonchallenged IFN-γ0 mice. Furthermore, swelling of the hind paws was not detected in vaccinated IFN-γ0 mice injected with BSK medium or dead spirochetes. Only viable B. burgdorferi organisms elicited the arthritis. When these studies were repeated three times with five mice per group, similar results were obtained.
FIG. 1.
Development of swelling in the hind paws of vaccinated (solid line) and nonvaccinated (broken line) IFN-γ0 mice with (▴) and without (○) challenge with B. burgdorferi. Data are reported as means and standard errors.
Confirmation of destructive arthritis by histopathologic examination.
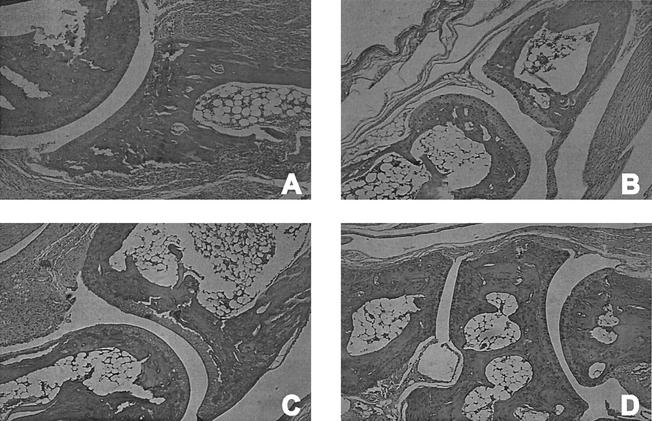
Vaccinated IFN-γ0 mice challenged with B. burgdorferi showed mild hypertrophic tenosynovitis at the knee joint and chronic inflammation involving the surrounding soft tissue, including skeletal muscle. The cartilage at the knee joint was irregular, and inflammatory cells were present in the joint space. The ankle joint showed pericapsular inflammation that extended along the long bone of the lower leg. In addition, there was erosion of the periosteum with pannus formation extending into the marrow space. The small bones of the foot showed extensive periarticular inflammation with bone erosion of the periosteum creating a scalloped appearance of the bones (Fig. 2A). In some areas, an adhesion membrane extended into the joint space, completely obliterating the articular cartilage and joint space. These changes collectively constitute prominent chronic destructive osteoarthropathy. In contrast, nonvaccinated mice challenged with B. burgdorferi showed a paucity of inflammation in the extremity, and the joints were free of significant pathologic findings (Fig. 2C). In addition, no evidence of inflammation or destruction of bony or articular surfaces was detected in nonchallenged, vaccinated mice (Fig. 2B) or nonvaccinated, nonchallenged IFN-γ0 animals (Fig. 2D).
FIG. 2.
Histopathologic findings in the hind paws of vaccinated (A and B) and nonvaccinated (C and D) IFN-γ0 mice with (A and C) and without (B and D) challenge with B. burgdorferi. Magnification, ×340 (original magnification, ×400).
Effects of TNF-α treatment on development of destructive Lyme arthritis.
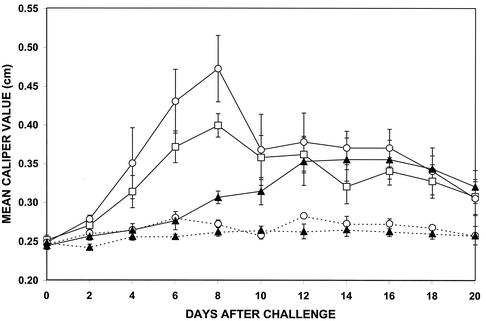
Three groups of five vaccinated IFN-γ0 mice each were challenged with 106 viable B. burgdorferi organisms 21 days after vaccination. Concomitantly, two of the three groups of vaccinated, challenged mice were treated with rTNF-α or anti-TNF-α on the day of challenge and daily thereafter for 7 days. Swelling of the hind paws was most severe in vaccinated, challenged mice treated with anti-TNF-α (Fig. 3). Swelling was detected on day 4 after challenge and peaked on day 8. In addition, the mobility of the mice was also affected. When treatment with anti-TNF-α was discontinued on day 8 after challenge, swelling of the hind paws gradually decreased and motility improved slightly. Less swelling of the hind paws was detected in vaccinated, challenged mice that received no anti-TNF-α treatment. Although the onset, peak, and resolution of swelling paralleled those detected in vaccinated, challenged mice treated with anti-TNF-α, the level of swelling of the hind paws was significantly lower (P < 0.01). In contrast, no swelling of the hind paws was detected in vaccinated, challenged mice during the period of treatment with rTNF-α. When treatment with rTNF-α was discontinued, slight swelling of the hind paws developed and persisted for the duration of the study. No swelling of the hind paws was detected in vaccinated, nonchallenged mice treated with either rTNF-α or anti-TNF-α.
FIG. 3.
Development of swelling in the hind paws of vaccinated IFN-γ0 mice with (solid line) and without (broken line) challenge with B. burgdorferi and treated with rTNF-α (▴) or anti-TNF-α (○). The remaining vaccinated, challenged group (□) did not receive anti-TNF-α treatment. Data are reported as means and standard errors.
Histopathologic examination of TNF-α-treated vaccinated, challenged mice.
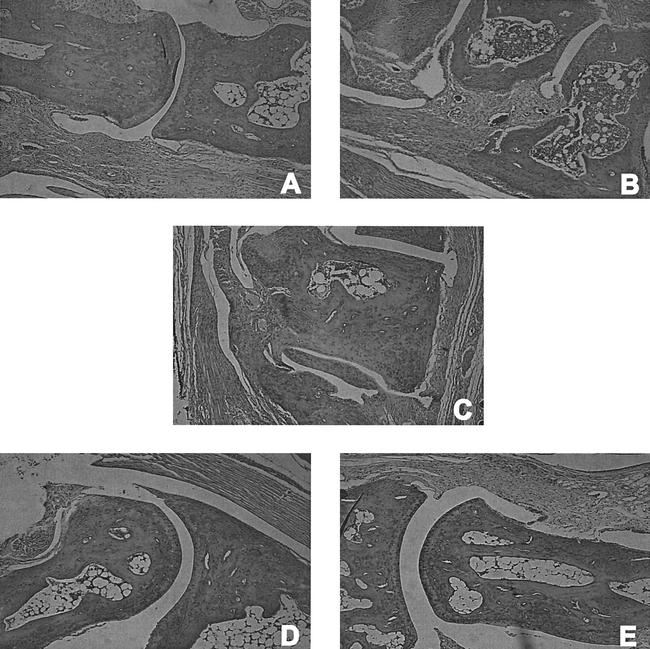
Vaccinated IFN-γ0 mice challenged with B. burgdorferi showed diffuse swelling of the hind paws resulting from fibroblastic proliferation and edematous changes of the soft tissue. Thickened synovium was observed in the tibiotarsal joint and along the small bones of the foot, which also exhibited destructive bone erosion (Fig. 4C). Vaccinated mice challenged with B. burgdorferi and treated with anti-TNF-α (Fig. 4B) showed severe fibroblastic proliferation throughout the soft tissues. Synovial thickening also occurred along all of the bones of the foot and ankle and descended from the tibia to the knee joint. Severe destructive bone erosion and osteoarthropathy of the small bones of the foot were present. Vaccinated mice challenged with B. burgdorferi and treated with rTNF-α (Fig. 4A) showed mild periarticular inflammation with mild hypertrophic tenosynovitis in the ankle. The small bones of the foot showed similar pericapsular inflammation with minimal erosion of the periosteum. The dermis of the lower foot showed extensive inflammation. Vaccinated, nonchallenged mice treated with either rTNF-α (Fig. 4D) or anti-TNF-α (Fig. 4E) exhibited no erosion of cartilage or bones, and significant inflammation was absent.
FIG. 4.
Histopathologic findings in the hind paws of vaccinated IFN-γ0 mice with (A, B, and C) and without (D and E) challenge with B. burgdorferi and treated with rTNF-α (A and D) or anti-TNF-α (B and E). Magnification, ×340 (original magnification, ×400).
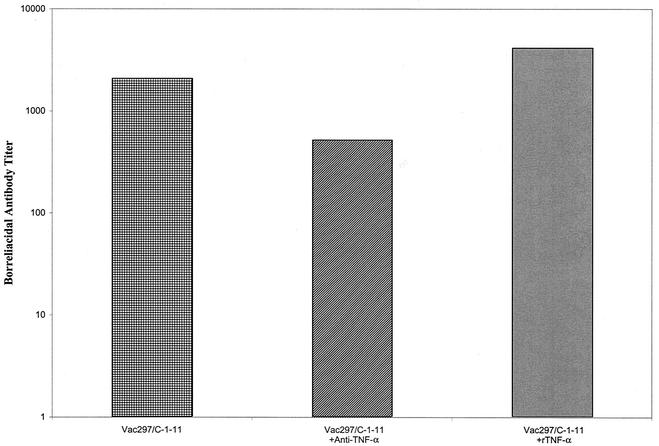
Effects of rTNF-α and anti-TNF-α on production of borreliacidal antibody.
Serum samples were obtained from vaccinated mice either not treated or treated with rTNF-α or anti-TNF-α 7, 14, and 21 days after challenge with B. burgdorferi and pooled. No differences were detected in the levels of borreliacidal antibody (titers, <160) among the groups at day 7 or 14 after challenge. However, a fourfold decrease in the level of borreliacidal antibody was detected in serum samples obtained on day 21 from vaccinated, challenged mice treated with anti-TNF-α (Fig. 5) compared to the level of borreliacidal antibody in non-TNF-α-treated vaccinated, challenged animals. Treatment of vaccinated, challenged mice with rTNF-α increased borreliacidal antibody production twofold compared to the control.
FIG. 5.
Titers of borreliacidal antibody in serum of vaccinated IFN-γ0 mice challenged with B. burgdorferi and not treated (left bar) or treated with rTNF-α (right bar) or anti-TNF-α (middle bar). When these studies were repeated, similar results were obtained.
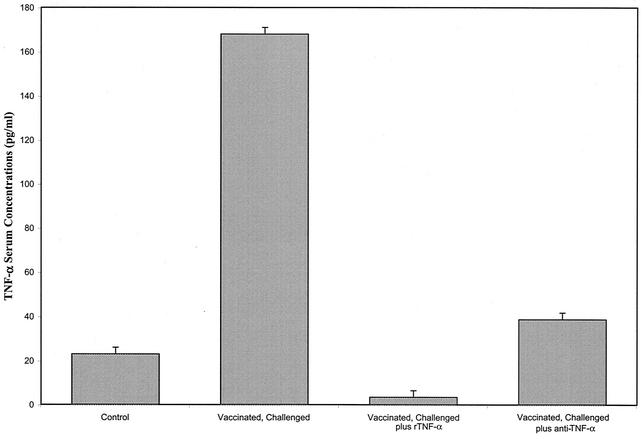
Serum TNF-α levels.
Serum samples were obtained from vaccinated, challenged IFN-γ0 mice either not treated or treated with anti-TNF-α or rTNF-α 24 h after the termination of treatment. Figure 6 shows that elevated levels of TNF-α were detected in the serum of non-treated vaccinated, challenged IFN-γ0 mice. Treatment of vaccinated, challenged IFN-γ0 mice with either anti-TNF-α or rTNF-α decreased the levels of TNF-α in serum. By day 21 after challenge (13 days after the termination of treatment with rTNF-α or anti-rTNF-α), the levels of TNF-α in the serum of these mice were similar to those in the serum of non-TNF-α-treated vaccinated, challenged mice.
FIG. 6.
Concentrations of TNF-α in sera of vaccinated, challenged IFN-γ0 mice either not treated or treated with rTNF-α or anti-TNF-α and in the sera of nonvaccinated, nonchallenged mice (control). Sera were obtained from vaccinated, challenged mice 24 h after termination of TNF-α treatments. Error bars indicate standard errors.
DISCUSSION
The term “arthritis” has been used to describe the inflammation of the joints and the hind paw swelling that genetically susceptible mice develop after infection with B. burgdorferi (3-5). The arthritis is nonosseous, and the inflammation is mild and limited to the synovial lining, subsynovial tissue, tendons, and muscle (3-5). When IFN-γ0 mice are infected with B. burgdorferi, a more severe arthritis develops (6). We report that B. burgdorferi-vaccinated IFN-γ0 mice challenged with B. burgdorferi developed extensive joint degenerative changes. Moreover, when B. burgdorferi-vaccinated IFN-γ0 mice challenged with B. burgdorferi were treated with anti-TNF-α, the severity of the destructive osteoarthropathy was enhanced and affected the mobility of the animals. In addition, extensive swelling of the hind paws occurred. In contrast, treatment of B. burgdorferi-vaccinated IFN-γ0 mice with rTNF-α inhibited the development of arthritis, including swelling of the hind paws.
This study constitutes the first documentation that vaccine-induced destructive Lyme osteoarthritis can be detected in mice. The signs of destructive arthritis were observed in B. burgdorferi-vaccinated IFN-γ0 mice challenged with the Lyme spirochete. Challenge with killed spirochetes failed to induce arthritis. The mice developed extensive erosion of the periosteum with pannus formation extending into the marrow space. The small bones of the foot also developed a scalloped appearance due to extensive erosion of the periosteum. In addition, the tibiotarsal joint displayed chronic hypertrophy and hyperplasia characterized by erosion of the articular cartilage and focal destruction of bone. This novel finding for IFN-γ0 mice confirms previous results obtained for hamsters (27), although the hamsters were not IFN-γ0. Hamsters vaccinated with a whole-cell preparation of formalin-inactivated B. burgdorferi or a subunit vaccine composed of OspA in alum developed severe destructive arthritis when challenged with infectious isolates of B. burgdorferi (11). The similar findings in hamsters and IFN-γ0 mice strengthen the likelihood that vaccination of humans may also cause arthritis. The immunologic events responsible for B. burgdorferi vaccine-associated arthritis will be easier to determine with the mouse model because of the widespread availability of appropriate reagents.
It has been hypothesized that IFN-γ plays a major role in the induction and expression of Lyme arthritis (18, 25, 26, 28, 44). However, Brown and Reiner (6) conclusively showed that IFN-γ is not required for increased susceptibility of mice to arthritis. When IFN-γ0 mice were challenged with B. burgdorferi, no difference in the severity of disease was detected between IFN-γ0 mice and mice of the parental strain, which had normal IFN-γ expression. We found that the severity of the arthritis was greatly augmented when IFN-γ0 mice were vaccinated and then challenged with B. burgdorferi. Our present data suggest that TNF-α plays a major role in the expression of destructive osteoarthritis in B. burgdorferi-vaccinated IFN-γ0 mice challenged with B. burgdorferi. TNF-α is known to be centrally involved in the control of infectious agents, inflammation, and autoimmune phenomena (1, 29). TNF-α also affects cartilage degradation and bone resorption (15, 33, 35, 39). Moreover, TNF-α contributes to tissue damage by induction of the release of tissue-damaging enzymes from synovial cells and articular chrondrocytes and specifically activates osteoclasts (2, 15, 35, 39).
Our findings showed that treatment with anti-TNF-α actually augmented the severity of the destructive arthritis detected in vaccinated IFN-γ0 mice challenged with B. burgdorferi. Vaccinated, challenged IFN-γ0 mice developed hind paw swelling (caliper value [mean and standard error], 0.39 ± 0.01 cm) that peaked on day 7. However, treatment of these animals with anti-TNF-α further enhanced the severity of hind paw swelling (caliper value, 0.47 ± 0.04 cm). Histopathologic examination confirmed that treatment with anti-TNF-α caused extensive pathologic findings, with considerable destruction of bone and cartilage. In contrast, treatment with rTNF-α appeared to inhibit the development of destructive arthritis. Fewer histopathologic findings were observed in these mice than in the non-rTNF-α-treated vaccinated, challenged IFN-γ0 mice.
We found these results surprising. In general, TNF-α is a key cytokine in driving the arthritic inflammatory response (9, 12, 15, 33, 35, 38, 40), especially rheumatoid arthritis (9, 21). Neutralization of TNF-α prevents the development of arthritis or ameliorates disease activity (23, 31, 40, 42, 43). In contrast, we show that treatment with anti-TNF-α exacerbates the development of destructive Lyme disease-associated arthritis, while rTNF-α treatment limits the development of this form of arthritis. These results suggest that TNF-α down-regulates the development of destructive Lyme osteoarthritis. Our results support the findings of others (45) that treatment with TNF-α can limit the pathologic response of the host to infectious agents.
The exact mechanism(s) by which anti-TNF-α or rTNF-α exerts its influence on the development of experimental vaccine-induced destructive Lyme osteoarthritis remains unclear. Our results show that treatment with anti-TNF-α or rTNF-α affects the levels of borreliacidal antibody. Treatment of vaccinated, challenged IFN-γ0 mice with rTNF-α increased the production of borreliacidal antibody. It is possible that borreliacidal antibody acts to reduce the challenge inoculum and thereby prevents the induction of arthritis. By the same token, the fourfold decrease in the levels of borreliacidal antibody produced in anti-TNF-α-treated vaccinated, challenged IFN-γ0 mice may have resulted in a failure to kill B. burgdorferi and prevent the induction of severe destructive arthritis. It is known that small changes in the levels of borreliacidal antibody can affect the induction of arthritis (22, 27) and protection against infection (8, 13, 30, 34). An alternative explanation is dependent on the effect of TNF-α on other effector molecules. High levels of TNF-α are known to stimulate cortisol production, which could suppress the expression of arthritis (9, 41). In support of this mechanism, we showed that discontinuation of treatment with rTNF-α allowed the development of destructive arthritis. When the levels of TNF-α are decreased by treatment with anti-TNF-α, other proinflammatory cytokines or biological mediators of arthritis may exert their adverse effects. We showed that the termination of anti-TNF-α treatment decreased the severity of the arthritic response.
Finally, a combination of these mechanisms may have occurred. We detected elevated levels of TNF-α in serum samples from vaccinated, challenged IFN-γ0 mice. Elevated levels of TNF-α were also detected in supernatants of cultures of lymph node cells obtained from these mice after incubation with B. burgdorferi. When these mice were treated with either anti-TNF-α or rTNF-α, the levels of serum TNF-α were significantly decreased. The low levels of serum TNF-α after treatment of vaccinated, challenged IFN-γ0 mice with anti-TNF-α is understandable. Blockage of TNF-α by anti-TNF-α may have prevented the processing of B. burgdorferi by phagocytic cells and the presentation of borrelial antigen for the production of borreliacidal antibody. Concomitantly, non-TNF-α-activated macrophages may have up-regulated other proinflammatory mediators for the induction of severe destructive Lyme arthritis. In contrast, the administration of rTNF-α may have activated macrophages (20) to reduce the spirochetal burden, enhanced the processing of B. burgdorferi for the production of borreliacidal antibody, stimulated cortisol production, and down-regulated the production of TNF-α for the induction of arthritis. When rTNF-α treatment was terminated, the levels of TNF-α in serum increased and destructive arthritis developed.
In conclusion, TNF-α is involved in the modulation of vaccine-induced experimental destructive Lyme osteoarthritis. Elevated levels of TNF-α in tissues inhibited the development of destructive arthritis, while low levels of TNF-α exacerbated the arthritic response. The mechanism by which TNF-α directly or indirectly exerts influence on the development of destructive arthritis is unknown. It is clear that the regulation mechanism does not require INF-γ. Studies are in progress to determine the effects of TNF-α on other proinflammatory cytokines that may be involved in the induction of destructive Lyme arthritis.
Acknowledgments
We are grateful to Chad A. Wieneke for helpful discussions and assistance and to Dean A. Jobe, Jennifer A. Marks, and Kerry A. Casey for technical assistance. In addition, we are grateful to Dean D. Manning and William P. Weidanz for critical evaluation of the manuscript.
REFERENCES
- 1.Adams, L. B., C. M. Mason, J. K. Kolls, D. Scollard, J. L. Krahenbuhl, and S. Nelson. 1995. Exacerbation of acute and chronic murine tuberculosis by administration of a tumor necrosis factor receptor-expressing adenovirus. J. Infect. Dis. 171:400-405. [DOI] [PubMed] [Google Scholar]
- 2.Arend, W. P., and J. M. Dayer. 1995. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor α in rheumatoid arthritis. Arthritis Rheum. 38:151-160. [DOI] [PubMed] [Google Scholar]
- 3.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 4.Barthold, S. W., and M. S. de Souza. 1995. Exacerbation of Lyme arthritis in beige mice. J. Infect. Dis. 172:778-784. [DOI] [PubMed] [Google Scholar]
- 5.Barthold, S. W., M. S. de Souza, J. L. Janotka, A. L. Smith, and D. H. Persing. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:951-971. [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, C. R., and S. L. Reiner. 1999. Experimental Lyme arthritis in the absense of interleukin-4 or gamma interferon. Infect. Immun. 67:3329-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callister, S. M., K. L. Case, W. A. Agger, R. F. Schell, R. C. Johnson, and J. L. E. Ellingson. 1990. Effects of bovine serum albumin on the ability of Barbour-Stoenner-Kelly medium to detect Borrelia burgdorferi. J. Clin. Microbiol. 28:363-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callister, S. M., R. F. Schell, K. L. Case, S. D. Lovrich, and S. P. Day. 1992. Characterization of the borreliacidal antibody response to Borrelia burgdorferi in humans: a serodiagnostic test. J. Infect. Dis. 167:158-164. [DOI] [PubMed] [Google Scholar]
- 9.Choy, E. H. S., and G. S. Panayi. 2001. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 344:907-916. [DOI] [PubMed] [Google Scholar]
- 10.Coyle, P. K. 1993. Lyme disease, p. 179-183. In S. Manning (ed.), Pathogenesis of Lyme disease. Mosby-Year Book, St. Louis, Mo.
- 11.Croke, C. L., E. L. Munson, S. D. Lovrich, J. A. Christopherson, M. C. Remington, D. M. England, S. M. Callister, and R. F. Schell. 2000. Occurrence of severe destructive Lyme arthritis in hamsters vaccinated with outer surface protein A and challenged with Borrelia burgdorferi. Infect. Immun. 68:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeFosse, D. L., and R. C. Johnson. 1992. In vitro and in vivo induction of tumor necrosis factor alpha by Borrelia burgdorferi. Infect. Immun. 60:1109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fikrig, E., L. K. Bockenstedt, S. W. Barthold, M. Chen, H. Tao, P. Ali-Salaam, S. R. Telford, and R. A. Flavell. 1994. Sera from patients with chronic Lyme disease protect mice from Lyme borreliosis. J. Infect. Dis. 169:568-574. [DOI] [PubMed] [Google Scholar]
- 14.Ganapamo, F., V. A. Dennis, and M. T. Philipp. 2000. Early induction of gamma interferon and interleukin-10 production in draining lymph nodes from mice infected with Borrelia burgdorferi. Infect. Immun. 68:7162-7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girasole, G., G. Passeri, R. L. Jilka, and S. C. Manolagas. 1991. Interleukin-11: a new cytokine critical for osteoclast development. J. Clin. Investig. 93:1516-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross, D. M., A. C. Steere, and B. T. Huber. 1998. T helper 1 response is dominant and localized to the synovial fluid in patients with Lyme arthritis. J. Immunol. 160:1022-1028. [PubMed] [Google Scholar]
- 17.Gross, D. M., T. Forsthuber, M. Tary-Lehmann, C. Etling, K. Ito, Z. A. Nagy, J. A. Field, A. C. Steere, and B. Hubner. 1998. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science 281:703-706. [DOI] [PubMed] [Google Scholar]
- 18.Harjacek, M., S. Diaz-Cano, B. A. Alman, J. Coburn, R. Ruthazer, H. Wolfe, and A. C. Steere. 2000. Prominent expression of mRNA for proinflammatory cytokines in synovium in patients with juvenile rheumatoid arthritis or chronic Lyme arthritis. J. Rheumatol. 27:497-503. [PubMed] [Google Scholar]
- 19.Hayes, M. P., S. L. Freeman, and R. P. Donnelly. 1995. IFN-gamma priming of monocytes enhances LPS-induced TNF production by augmenting both transcription and MRNA stability. Cytokine 7:427-435. [DOI] [PubMed] [Google Scholar]
- 20.Held, T. K., X. Weihua, L. Yuan, D. V. Kalvakolanu, and A. S. Cross. 1999. Gamma interferon augments macrophage activation lipopolysaccharide by two distinct mechanisms, at the signal transduction level and via an autocrine mechanism involving tumor necrosis factor alpha and interleukin-1. Infect. Immun. 67:206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houssiau, F. A. 1995. Cytokines in rheumatoid arthritis. Clin. Rheumatol. 14(Suppl. 2):10-13. [DOI] [PubMed] [Google Scholar]
- 22.Jobe, D. A., S. A. Callister, L. C. L. Lim, S. D. Lovrich, and R. F. Schell. 1994. Ability of canine Lyme disease vaccine to protect hamsters against infection with several isolates of Borrelia burgdorferi. J. Clin. Microbiol. 32:618-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joosten, L. A. B., M. M. A. Helsen, F. A. J. van de Loo, and W. B. van den Berg. 1996. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice. A comparative study using anti-TNFα, anti-IL-1α/β and anti-IL-1Rα. Arthritis Rheum. 39:797-809. [DOI] [PubMed] [Google Scholar]
- 24.Kalish, R. A., J. M. Leong, and A. C. Steere. 1993. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect. Immun. 61:2774-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keane-Myers, A., and S. P. Nickell. 1995. T cell subset-dependent modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 154:1770-1776. [PubMed] [Google Scholar]
- 26.Keane-Myers, A., and S. P. Nickell. 1995. Role of IL-4 and IFN-γ in modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 155:2020-2028. [PubMed] [Google Scholar]
- 27.Lim, L. C. L., D. M. England, B. K. DuChateau, N. J. Glowacki, J. R. Creson, S. D. Lovrich, S. M. Callister, D. A. Jobe, and R. F. Schell. 1994. Development of destructive arthritis in vaccinated hamsters challenged with Borrelia burgdorferi. Infect. Immun. 62:2825-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matyniak, J. E., and S. L. Reiner. 1995. T helper phenotype and genetic susceptibility in experimental Lyme disease. J. Exp. Med. 181:1251-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohan, V. P., C. A. Scanga, K. Yu, H. M. Scott, K. E. Tanaka, E. Tsang, M. C. Tsai, J. L. Flynn, and J. Chan. 2001. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect. Immun. 69:1847-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Padilla, M. L., S. M. Callister, R. F. Schell, G. L. Bryant, D. A. Jobe, S. D. Lovrich, B. K. Du Chateau, and J. R. Jensen. 1996. Characterization of the protective borreliacidal antibody in humans and hamsters after vaccination with a Borrelia burgdorferi outer surface protein A vaccine. J. Infect. Dis. 174:739-749. [DOI] [PubMed] [Google Scholar]
- 31.Piquet, P. F., G. E. Grau, G. Vesin, H. Loetscher, R. Gentz, and W. Leslauer. 1992. Evolution of collagen arthritis in mice is arrested by treatment with anti-tumor necrosis factor (TNF) antibody or a recombinant soluble TNF receptor. Immunology 77:510-514. [PMC free article] [PubMed] [Google Scholar]
- 32.Rose, C. D., P. T. Fawcett, and K. M. Gibney. 2001. Arthritis following recombinant ospA vaccination for Lyme disease. J. Rheumatol. 28:2555-2557. [PubMed] [Google Scholar]
- 33.Saez-Llorens, X., H. S. Jafari, K. D. Olsen, H. Nariuchi, E. J. Hansen, and G. H. McCracken, Jr. 1991. Induction of suppurative arthritis in rabbits by Haemophilus endotoxin, tumor necrosis factor-α, and interleukin-1β. J. Infect. Dis. 163:1267-1272. [DOI] [PubMed] [Google Scholar]
- 34.Schmitz, J. L., R. F. Schell, S. D. Lovrich, S. M. Callister, and J. E. Coe. 1991. Characterization of the protective antibody response to Borrelia burgdorferi in experimentally infected LSH hamsters. Infect. Immun. 59:1916-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shingu, M., Y. Nagai, T. Isayama, T. Naono, M. Nobunaga, and Y. Nagai. 1993. The effects of cytokines on metalloproteinase inhibitors (TIMP) and collagenase production by human chrondrocytes and TIMP production by synovial cells and endothelial cells. Clin. Exp. Immunol. 94:145-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steere, A. C., A. Grbofsky, M. E. Patarroyo, F. J. Winchester, J. A. Hardin, and S. E. Malawista. 1979. Chronic Lyme arthritis: clinical and immunogenetic differentiation from rheumatoid arthritis. Ann. Intern. Med. 90:896-901. [DOI] [PubMed] [Google Scholar]
- 37.Steere, A. C., R. T. Schoen, and E. Taylor. 1987. The clinical evolution of Lyme arthritis. Ann. Intern. Med. 107:725-731. [DOI] [PubMed] [Google Scholar]
- 38.Talkington, J., and S. P. Nickell. 1999. Borrelia burgdorferi spirochetes induce mast cell activation and cytokine release. Infect. Immun. 67:1107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas, B. M., G. R. Mundy, and J. J. Chambers. 1987. Tumor necrosis factor alpha and beta induce osteoblastic cells to stimulate osteoclast bone resorption. J. Immunol. 138:775-779. [PubMed] [Google Scholar]
- 40.Thorbecke, G. J., R. Shah, C. H. Leu, A. P. Kuruvilla, A. M. Haridson, and M. A. Palladino. 1992. Involvement of endogenous tumor necrosis factor α and transforming growth factor β during induction of collagen type II arthritis in mice. Proc. Natl. Acad. Sci. USA 89:7375-7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tilders, F. J., R. H. DeRijk, A. M. Van Dam, V. A. Vincent, K. Schotanus, and J. H. Persoons. 1994. Activation of the hypothalamus-pituitary-adrenal axis by bacterial endotoxins: routes and intermediate signals. Psychoneuroendocrinology. 19:209-232. [DOI] [PubMed] [Google Scholar]
- 42.Williams, R. O., M. Feldman, and R. N. Maini. 1992. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 89:9784-9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wooley, P. H., J. Dutcher, M. B. Widmer, and S. Gills. 1993. Influence of a recombinant human soluble tumor necrosis factor receptor Fc fusion protein on type II collagen-induced arthritis in mice. J. Immunol. 151:6602-6607. [PubMed] [Google Scholar]
- 44.Yssel, H., M.-C. Shanafelt, C. Sodoerberg, P. V. Schneider, J. Anzola, and G. Peltz. 1991. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J. Exp. Med. 174:593-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeidner, N., M. Dreitz, D. Belasco, and D. Fish. 1996. Suppression of acute Ixodes scapularis-induced Borrelia burgdorferi infection using tumor necrosis factor-α, interleukin-2, and interferon-γ. J. Infect. Dis. 173:187-195. [DOI] [PubMed] [Google Scholar]