Abstract
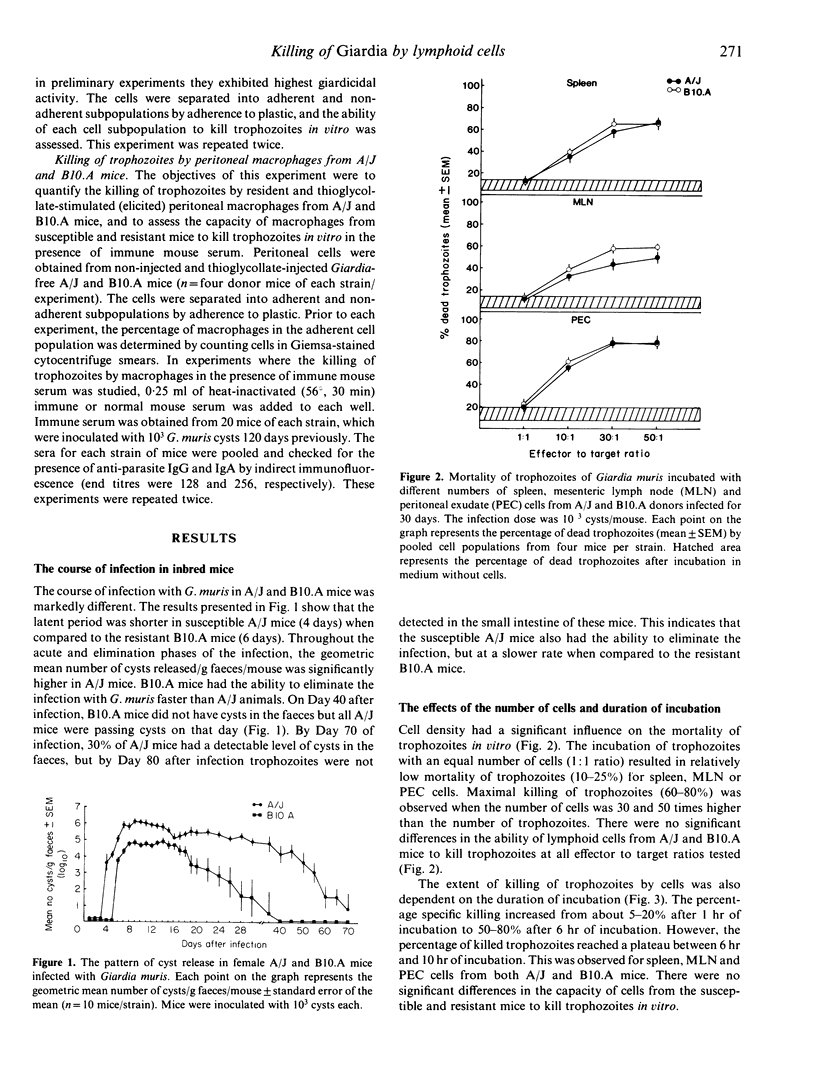
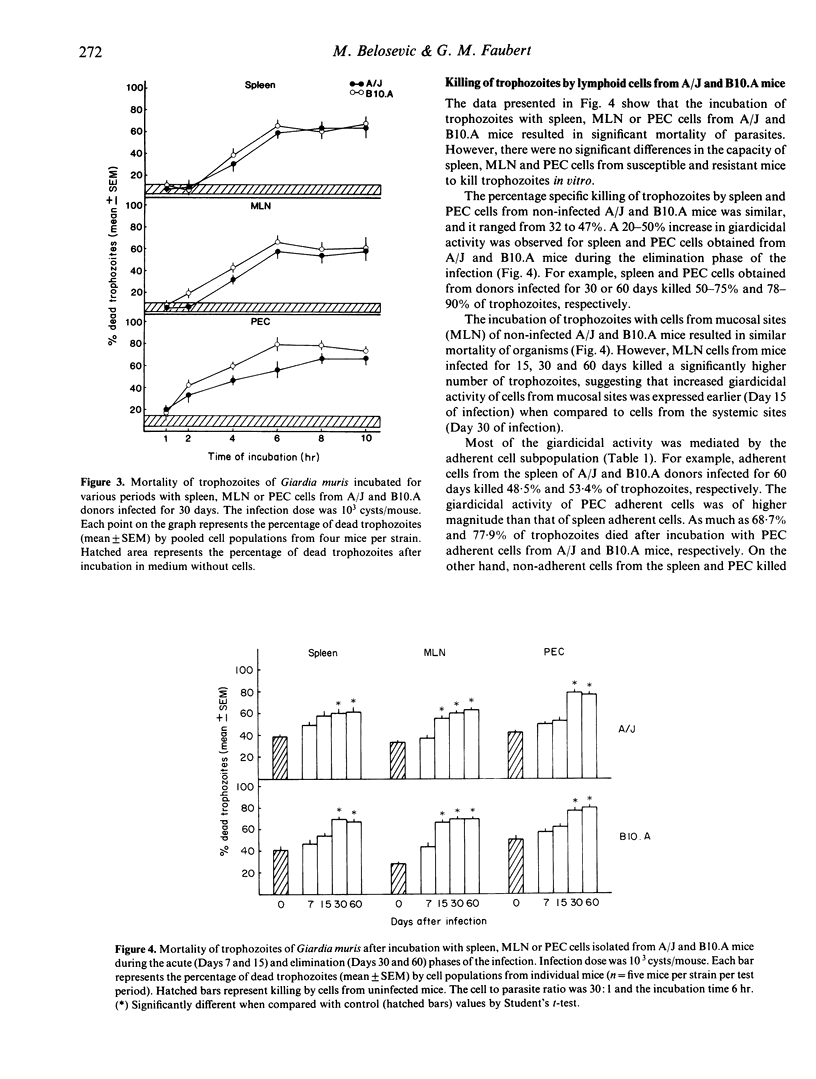
The ability of spleen, mesenteric lymph node (MLN) and peritoneal exudate (PEC) cells from susceptible (A/J) and resistant (B10.A) mice to kill trophozoites in vitro was determined. Both duration of incubation and cell density influenced giardicidal activity. Maximal killing was observed after 6 hr of incubation at the effector to target ratios of 30:1 and 50:1. Cells isolated from A/J and B10.A mice during the elimination phase of the infection killed more trophozoites than those isolated from mice during the acute phase of the infection. Cells isolated from mucosal sites (MLN) of donors infected for 15 days killed more trophozoites in vitro than those isolated from systemic sites (spleen, PEC). There were no differences in the giardicidal activity of cells from susceptible and resistant mice. Killing of trophozoites was mediated by plastic-adherent cells with macrophage properties. Non-specific stimulation with thioglycollate and the presence of immune mouse serum enhanced the capacity of macrophages to kill parasites. There was no apparent relationship between the capacity of A/J and B10.A mice to mount cell-mediated effector responses and their ability to control the infection with Giardia muris.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belosevic M., Faubert G. M. Giardia muris: correlation between oral dosage, course of infection, and trophozoite distribution in the mouse small intestine. Exp Parasitol. 1983 Aug;56(1):93–100. doi: 10.1016/0014-4894(83)90100-5. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Faubert G. M., MacLean J. D. The effects of cyclosporin A on the course of infection with Giardia muris in mice. Am J Trop Med Hyg. 1986 May;35(3):496–500. doi: 10.4269/ajtmh.1986.35.496. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Faubert G. M., Skamene E., MacLean J. D. Susceptibility and resistance of inbred mice to Giardia muris. Infect Immun. 1984 May;44(2):282–286. doi: 10.1128/iai.44.2.282-286.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belosevic M., Faubert G. M. Temporal study of acquired resistance in infections of mice with Giardia muris. Parasitology. 1983 Dec;87(Pt 3):517–524. doi: 10.1017/s0031182000083037. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Mahmoud A. A. Killing of schistosomula of Schistosoma mansoni by normal human monocytes. J Immunol. 1979 Aug;123(2):949–951. [PubMed] [Google Scholar]
- Gale R. P., Zighelboim J. Polymorphonuclear leukocytes in antibody-dependent cellular cytotoxicity. J Immunol. 1975 Mar;114(3):1047–1051. [PubMed] [Google Scholar]
- Kaplan B. S., Uni S., Aikawa M., Mahmoud A. A. Effector mechanism of host resistance in murine giardiasis: specific IgG and IgA cell-mediated toxicity. J Immunol. 1985 Mar;134(3):1975–1981. [PubMed] [Google Scholar]
- Kassis A. I., Aikawa M., Mahmoud A. F. Mouse antibody-dependent eosinophil and macrophage adherence and damage to schistosomula of Schistosoma mansoni. J Immunol. 1979 Feb;122(2):398–405. [PubMed] [Google Scholar]
- Lowell G. H., MacDermott R. P., Summers P. L., Reeder A. A., Bertovich M. J., Formal S. B. Antibody-dependent cell-mediated antibacterial activity: K lymphocytes, monocytes, and granulocytes are effective against shigella. J Immunol. 1980 Dec;125(6):2778–2784. [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):938–949. doi: 10.1084/jem.150.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S., Leonard E. J., Wyler D. J. Intracellular replication and lymphokine-induced destruction of Leishmania tropica in C3H/HeN mouse macrophages. J Immunol. 1981 Dec;127(6):2381–2386. [PubMed] [Google Scholar]
- Nair K. V., Gillon J., Ferguson A. Corticosteroid treatment increases parasite numbers in murine giardiasis. Gut. 1981 Jun;22(6):475–480. doi: 10.1136/gut.22.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. L., Allen C. L., Stevens D. P. Phagocytosis of Giardia muris by macrophages in Peyer's patch epithelium in mice. Infect Immun. 1981 Aug;33(2):591–601. doi: 10.1128/iai.33.2.591-601.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulescu S., Meyer E. A. Opsonization in vitro of Giardia lamblia trophozoites. Infect Immun. 1981 May;32(2):852–856. doi: 10.1128/iai.32.2.852-856.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Thomson I. C., Stevens D. P., Mahmoud A. A., Warren K. S. Acquired resistance to infection in an animal model of giardiasis. J Immunol. 1976 Nov;117(5 PT2):2036–2037. [PubMed] [Google Scholar]
- Smith P. D., Elson C. O., Keister D. B., Nash T. E. Human host response to Giardia lamblia. I. Spontaneous killing by mononuclear leukocytes in vitro. J Immunol. 1982 Mar;128(3):1372–1376. [PubMed] [Google Scholar]
- Smith P. D., Keister D. B., Wahl S. M., Meltzer M. S. Defective spontaneous but normal antibody-dependent cytotoxicity for an extracellular protozoan parasite, Giardia lamblia, by C3H/HeJ mouse macrophages. Cell Immunol. 1984 Apr 15;85(1):244–251. doi: 10.1016/0008-8749(84)90294-6. [DOI] [PubMed] [Google Scholar]
- Stevens D. P., Frank D. M., Mahmoud A. A. Thymus dependency of host resistance to Giardia muris infection: studies in nude mice. J Immunol. 1978 Feb;120(2):680–682. [PubMed] [Google Scholar]
- Tagliabue A., Befus A. D., Clark D. A., Bienenstock J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J Exp Med. 1982 Jun 1;155(6):1785–1796. doi: 10.1084/jem.155.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]


