Abstract
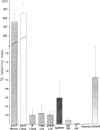
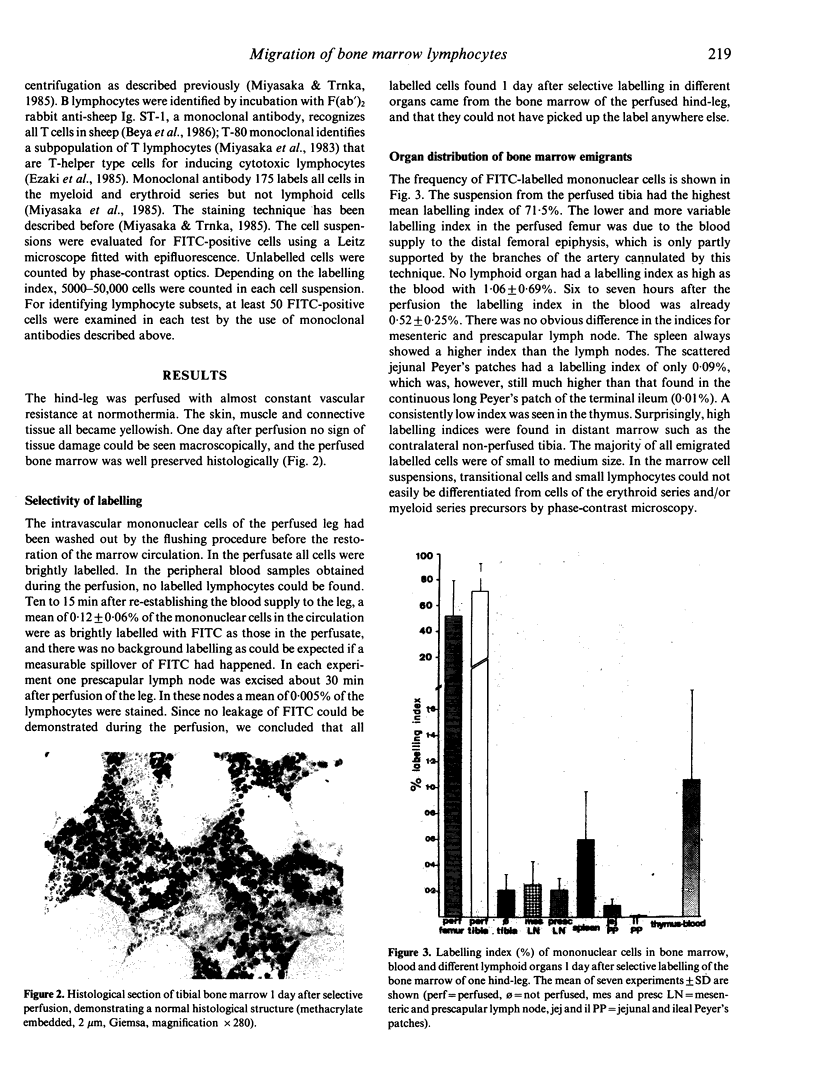
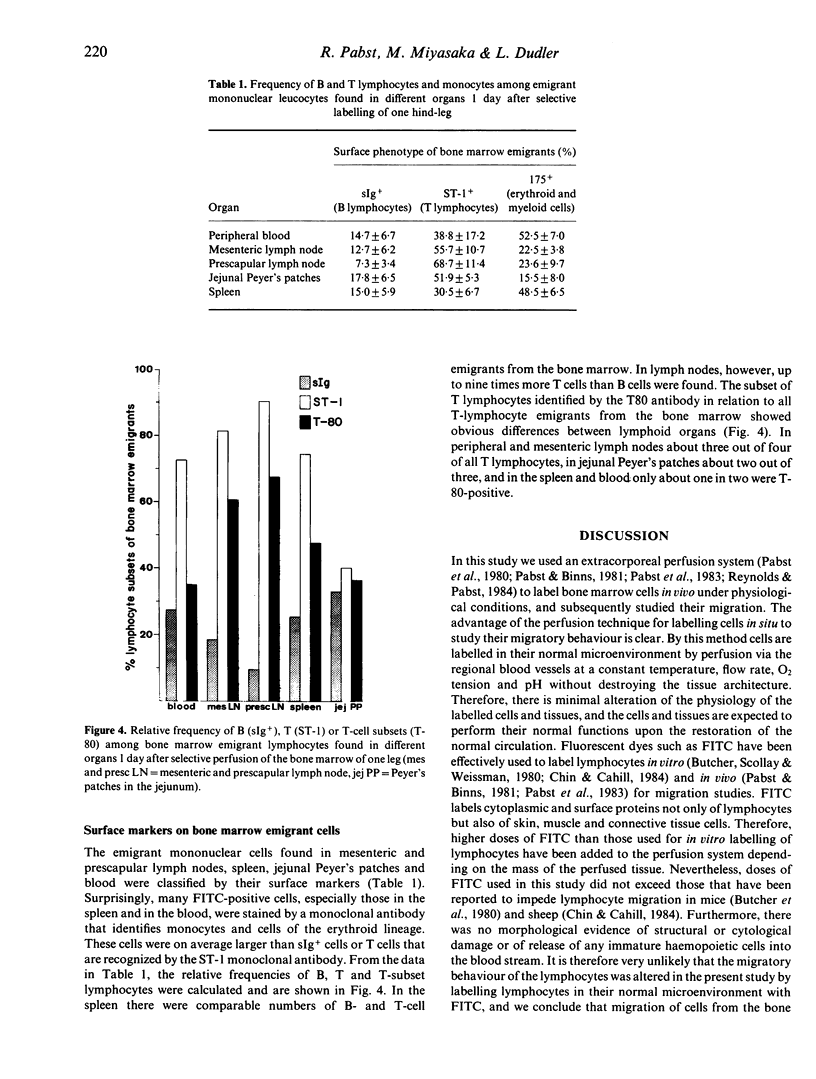
In normal young lambs the bone marrow was selectively labelled with fluorescein isothiocyanate by a temporary perfusion of one hind-leg. One day later, the incidence of bone marrow emigrants in different lymph nodes, spleen, Peyer's patches, thymus, non-perfused bone marrow and blood was determined. The emigrants were also phenotyped by the use of monoclonal antibodies and classified into monocytes or lymphocyte subsets. Large numbers of lymphocytes left the bone marrow of the perfused leg during 1 day. Considerable numbers of cells migrated to other bone marrow compartments. Varying numbers of mononuclear emigrants were found in peripheral lymphoid organs, with labelling indices ranging from 1.06% in the blood to 0.004% in the thymus. In the spleen, comparable numbers of B- and T-lymphocyte emigrants from the bone marrow were found, whereas in the blood, lymph nodes and jejunal Peyer's patches many more emigrants were T lymphocytes than B lymphocytes. In the prescapular lymph nodes, for instance, 90.4% of emigrants were T cells but only 9.6% were B cells. Based on the large numbers of lymphocytes emigrating from the bone marrow, their phenotypes and their entry into other bone marrow compartments, it it can be concluded that the bone marrow of young lambs is an integral part of the migratory route of lymphocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beya M. F., Miyasaka M., Dudler L., Ezaki T., Trnka Z. Studies on the differentiation of T lymphocytes in sheep. II. Two monoclonal antibodies that recognize all ovine T lymphocytes. Immunology. 1986 Jan;57(1):115–121. [PMC free article] [PubMed] [Google Scholar]
- Brenan M., Parish C. R. Intracellular fluorescent labelling of cells for analysis of lymphocyte migration. J Immunol Methods. 1984 Nov 16;74(1):31–38. doi: 10.1016/0022-1759(84)90364-8. [DOI] [PubMed] [Google Scholar]
- Butcher E. C., Scollay R. G., Weissman I. L. Direct fluorescent labeling of cells with fluorescein or rhodamine isothiocyanate. II. Potential application to studies of lymphocyte migration and maturation. J Immunol Methods. 1980;37(2):109–121. doi: 10.1016/0022-1759(80)90196-9. [DOI] [PubMed] [Google Scholar]
- Chin G. W., Cahill N. P. The appearance of fluorescein-labelled lymphocytes in lymph following in vitro or in vivo labelling: the route of lymphocyte recirculation through mesenteric lymph nodes. Immunology. 1984 Jun;52(2):341–347. [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. Mechanisms of corticosteroid action on lymphocyte subpopulations. I. Redistribution of circulating T and b lymphocytes to the bone marrow. Immunology. 1975 Apr;28(4):669–680. [PMC free article] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. Surface antigens, SBU-T4 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology. 1985 Aug;55(4):739–748. [PMC free article] [PubMed] [Google Scholar]
- Miyasaka M., Heron I., Dudler L., Cahill R. N., Forni L., Knaak T., Trnka Z. Studies on the differentiation of T lymphocytes in sheep. I. Recognition of a sheep T-lymphocyte differentiation antigen by a monoclonal antibody T-80. Immunology. 1983 Jul;49(3):545–553. [PMC free article] [PubMed] [Google Scholar]
- Mèly-Goubert B., Dudler L., Miyasaka M. Distribution of membrane-associated actin in sheep lymphocytes. Functional implications. Int Arch Allergy Appl Immunol. 1984;74(1):45–49. doi: 10.1159/000233514. [DOI] [PubMed] [Google Scholar]
- Osmond D. G. The contribution of bone marrow to the economy of the lymphoid system. Monogr Allergy. 1980;16:157–172. [PubMed] [Google Scholar]
- Pabst R., Binns R. M. In vivo labelling of the spleen and mesenteric lymph nodes with fluorescein isothiocyanate for lymphocyte migration studies. Immunology. 1981 Oct;44(2):321–329. [PMC free article] [PubMed] [Google Scholar]
- Pabst R., Kaatz M., Westermann J. In situ labelling of bone marrow lymphocytes with fluorescein isothiocyanate for lymphocyte migration studies in pigs. Scand J Haematol. 1983 Sep;31(3):267–274. doi: 10.1111/j.1600-0609.1983.tb00651.x. [DOI] [PubMed] [Google Scholar]
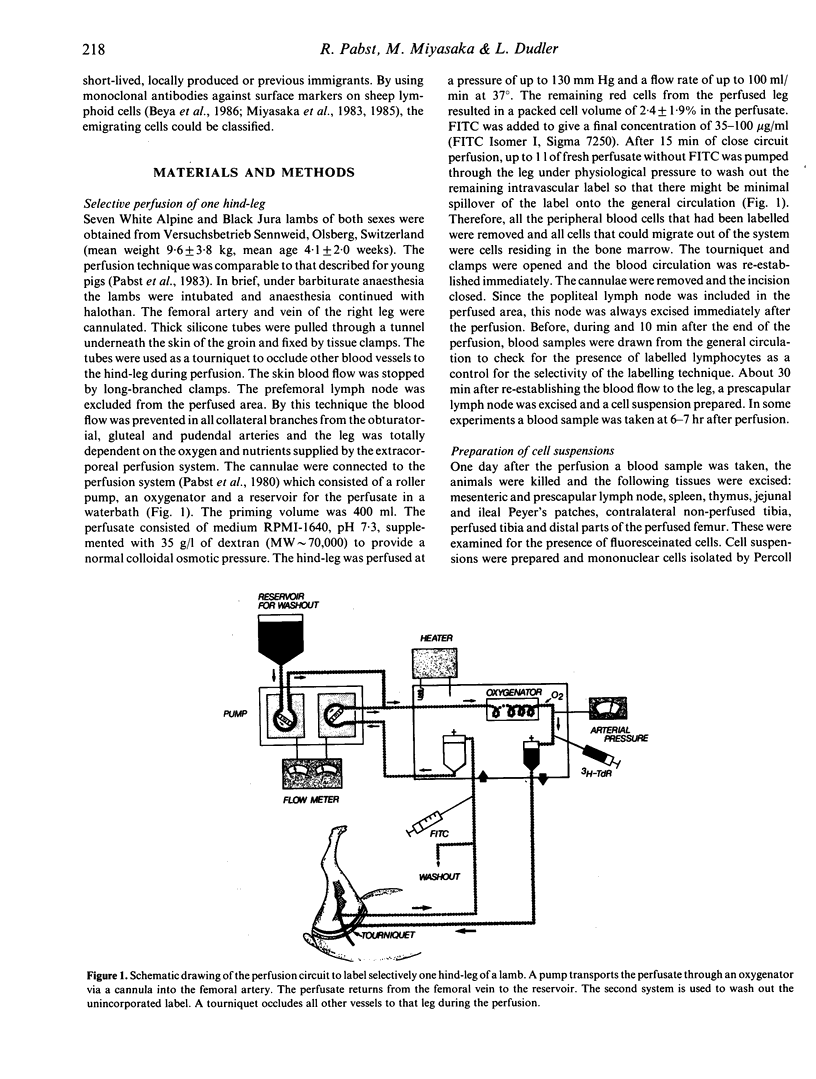
- Pabst R., Reilmann H., Neuhaus P. Selective labelling of lymphoid tissues by extracorporeal perfusion. J Immunol Methods. 1980;33(1):23–32. doi: 10.1016/0022-1759(80)90079-4. [DOI] [PubMed] [Google Scholar]
- Rannie G. H., Donald K. J. Estimation of the migration of thoracic duct lymphocytes to non-lymphoid tissues. A comparison of the distribution of radioactivity at intervals following i.v. transfusion of cells labelled with 3H, 14C, 75Se, 99mTc, 125I and 51Cr in the rat. Cell Tissue Kinet. 1977 Nov;10(6):523–541. [PubMed] [Google Scholar]
- Reynolds J. D., Pabst R. The emigration of lymphocytes from Peyer's patches in sheep. Eur J Immunol. 1984 Jan;14(1):7–13. doi: 10.1002/eji.1830140103. [DOI] [PubMed] [Google Scholar]
- Reynolds J., Pabst R., Bordmann G. Evidence for the existence of two distinct types of Peyer's patches in sheep. Adv Exp Med Biol. 1985;186:101–109. doi: 10.1007/978-1-4613-2463-8_12. [DOI] [PubMed] [Google Scholar]
- Röpke C., Hougen H. P., Everett N. B. Long-lived T and B lymphocytes in the bone marrow and thoracic duct lymph of the mouse. Cell Immunol. 1975 Jan;15(1):82–93. doi: 10.1016/0008-8749(75)90166-5. [DOI] [PubMed] [Google Scholar]
- Storb R., Thomas E. D. Allogeneic bone-marrow transplantation. Immunol Rev. 1983;71:77–102. doi: 10.1111/j.1600-065x.1983.tb01069.x. [DOI] [PubMed] [Google Scholar]