Abstract
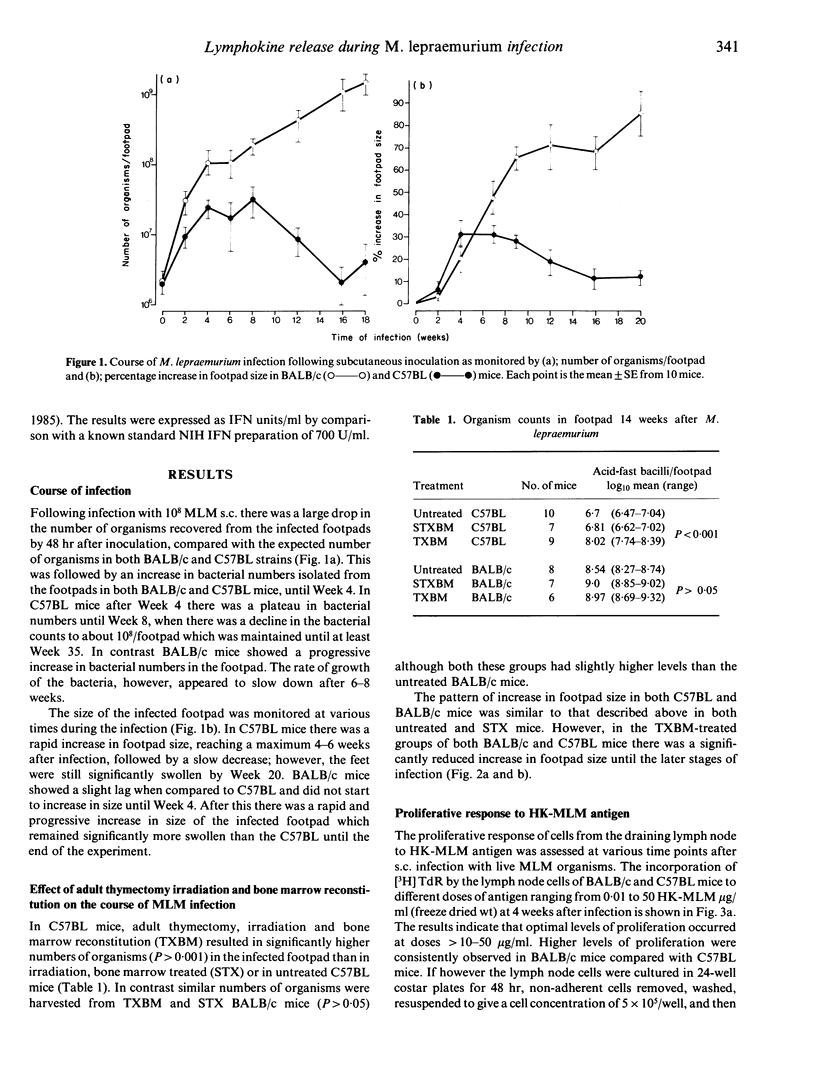
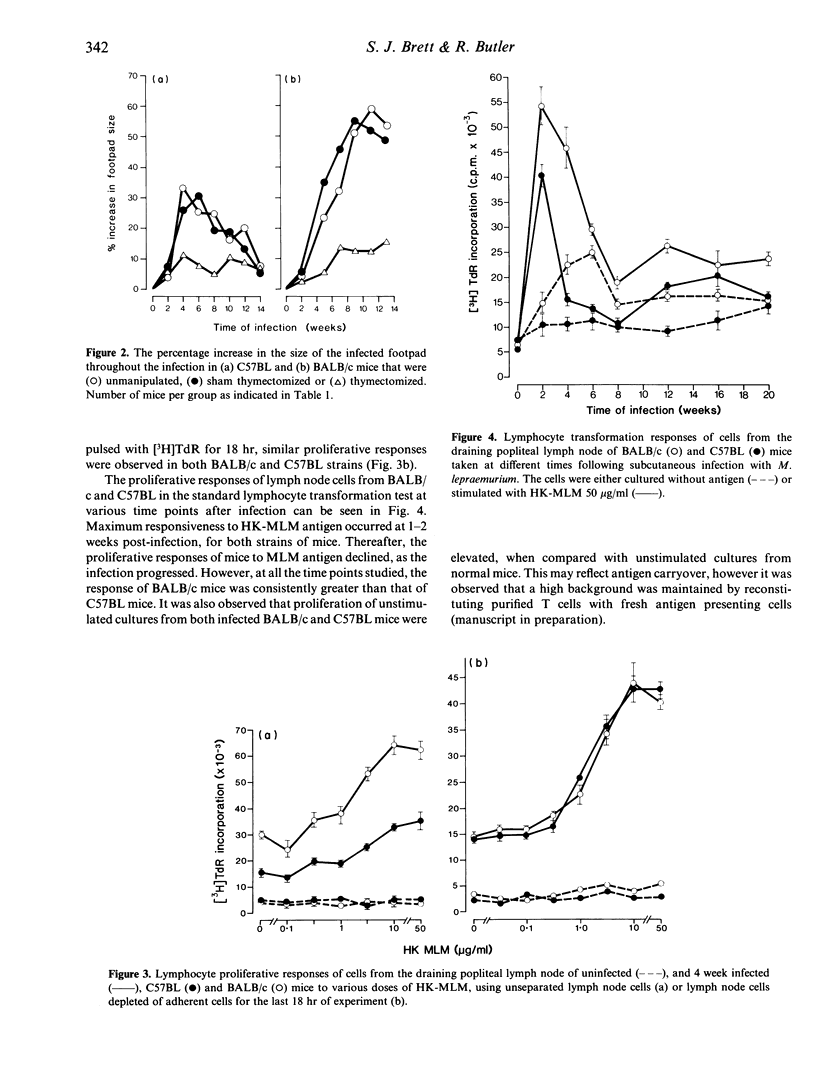
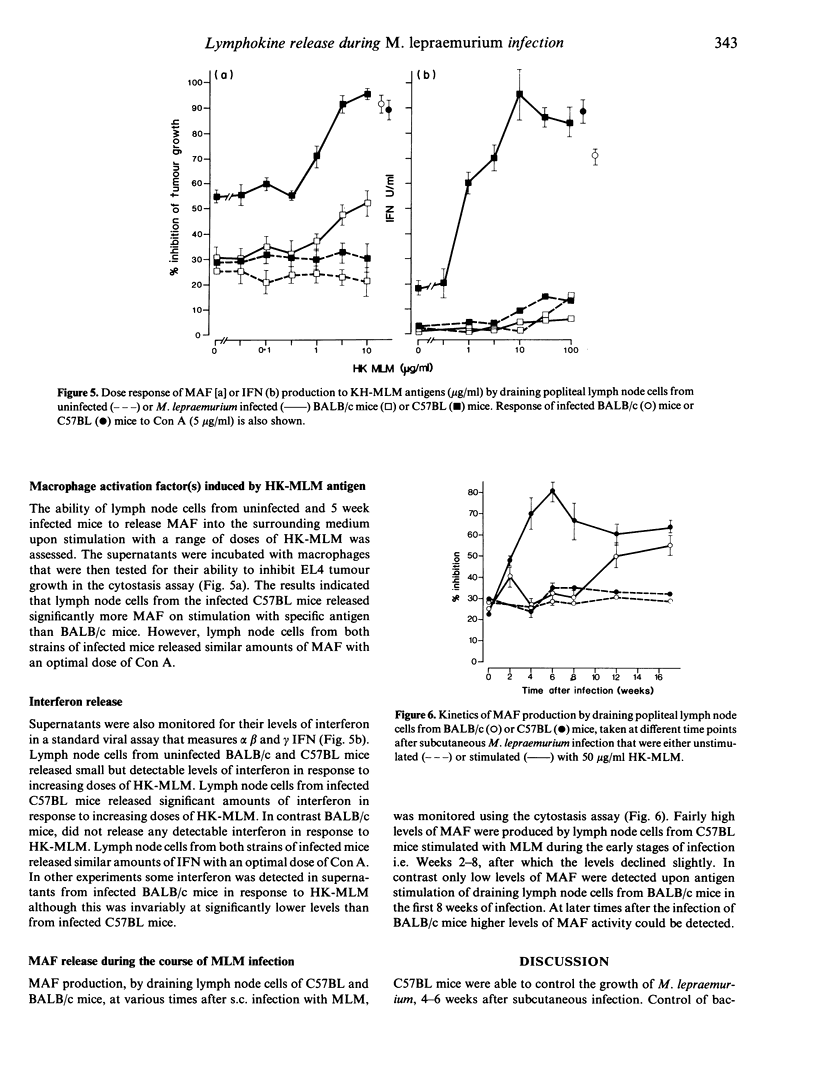
The kinetics of cell-mediated immunity developed during the course of Mycobacterium lepraemurium infection were determined in resistant (C57BL) and susceptible (BALB/c) mice. Control of M. lepraemurium growth following footpad infection was T-cell dependent in C57BL mice as shown by the finding that T-cell deprived mice had enhanced bacterial counts in the footpad. In contrast, T-cell deprivation did not significantly alter the course of infection in BALB/c mice. However a T-cell dependent inflammatory response, resulting in an increase in size of the infected footpad, occurred in both strains, although it developed slightly later in BALB/c mice. Cells isolated from the lymph nodes, draining the infected foot-pads, were assayed for their proliferative responses to heat-killed M. lepraemurium (HK-MLM) antigens. Although lymph node cells from both mouse strains proliferated to HK-MLM early in the infection (1-2 weeks) both C57BL and BALB/c mice developed diminished in vitro proliferative reactivity within 4-6 weeks post-infection. Supernatants derived from cultures of lymph-node cells that had been stimulated with concanavalin A (Con A) or HK-MLM antigens, were assayed for the presence of macrophage-activating factor (MAF) activity using a tumour cytostasis assay and interferon (IFN) activity using a viral growth inhibition assay. Significantly higher levels of MAF and IFN were found in culture supernatants deprived from HK-MLM stimulated lymph-node cells from infected C57BL mice than from BALB/c mice during the first 8 weeks of infection. However, cells from infected mice of both strains produced similar amounts of both MAF and IFN in response to Con A.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adu H. O., Curtis J., Turk J. L. The resistance of C57BL/6 mice to subcutaneous infection with Mycobacterium lepraemurium is dependent on both T cells and other cells of bone marrow origin. Cell Immunol. 1983 Jun;78(2):249–256. doi: 10.1016/0008-8749(83)90279-4. [DOI] [PubMed] [Google Scholar]
- Alexander J. Adoptive transfer of immunity and suppression by cells and serum in early Mycobacterium lepraemurium infections of mice. Parasite Immunol. 1979 Summer;1(2):159–166. doi: 10.1111/j.1365-3024.1979.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Alexander J., Curtis J. Development of delayed hypersensitivity responses in Mycobacterium lepraemurium infections in resistant and susceptible strains of mice. Immunology. 1979 Mar;36(3):563–567. [PMC free article] [PubMed] [Google Scholar]
- Alexander J., Kaye P. M. Immunoregulatory pathways in murine leishmaniasis: different regulatory control during Leishmania mexicana mexicana and Leishmania major infections. Clin Exp Immunol. 1985 Sep;61(3):674–682. [PMC free article] [PubMed] [Google Scholar]
- Alexander J., Smith C. C. Growth of Mycobacterium lepraemurium in nonstimulated and stimulated mouse peritoneal-derived and bone marrrow-derived macrophages in vitro. Infect Immun. 1978 Dec;22(3):631–636. doi: 10.1128/iai.22.3.631-636.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew P. W., Rees A. D., Scoging A., Dobson N., Matthews R., Whittall J. T., Coates A. R., Lowrie D. B. Secretion of a macrophage-activating factor distinct from interferon-gamma by human T cell clones. Eur J Immunol. 1984 Oct;14(10):962–964. doi: 10.1002/eji.1830141018. [DOI] [PubMed] [Google Scholar]
- Brett S. J. T-cell responsiveness in Mycobacterium lepraemurium infections in a "resistant" (CBA) and a "susceptible" (BALB/c) mouse strain. Cell Immunol. 1984 Nov;89(1):132–143. doi: 10.1016/0008-8749(84)90204-1. [DOI] [PubMed] [Google Scholar]
- Brown I. N., Krenzien H. N. Systemic Mycobacterium lepraemurium infection in mice: differences in doubling time in liver, spleen, and bone marrow, and a method for measuring the proportion of viable organisms in an inoculum. Infect Immun. 1976 Feb;13(2):480–486. doi: 10.1128/iai.13.2.480-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J., Adu H. O., Turk J. L. A lack of correlation between antigen-specific cellular reactions and resistance to Mycobacterium lepraemurium infection in mice. Immunology. 1981 Jun;43(2):293–301. [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Henson P. M., Campbell P. A. Enhanced accumulation of inflammatory neutrophils and macrophages mediated by transfer of T cells from mice immunized with Listeria monocytogenes. J Immunol. 1985 May;134(5):3449–3454. [PubMed] [Google Scholar]
- HART P. D., REES R. J. Effect of macrocyclon in acute and chronic pulmonary tuberculous infection in mice as shown by viable and total bacterial counts. Br J Exp Pathol. 1960 Aug;41:414–421. [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hockmeyer W. T., Walters D., Gore R. W., Williams J. S., Fortier A. H., Nacy C. A. Intracellular destruction of Leishmania donovani and Leishmania tropica amastigotes by activated macrophages: dissociation of these microbicidal effector activities in vitro. J Immunol. 1984 Jun;132(6):3120–3125. [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. III. Nature and significance of specific suppression of cell-mediated immunity in mice highly susceptible to Leishmania tropica. J Exp Med. 1980 Sep 1;152(3):594–607. doi: 10.1084/jem.152.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. IV. Prophylactic effect of sublethal irradiation as a result of abrogation of suppressor T cell generation in mice genetically susceptible to Leishmania tropica. J Exp Med. 1981 Mar 1;153(3):557–568. doi: 10.1084/jem.153.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Palfliet K. Strain variation in interferon gamma production of BCG-sensitized mice challenged with PPD. I. CBA/Ca mice are low producers in vivo, but high producers in vitro. Cell Immunol. 1983 Sep;80(2):329–334. doi: 10.1016/0008-8749(83)90121-1. [DOI] [PubMed] [Google Scholar]
- Kildahl-Andersen O., Nissen-Meyer J. The role of monocyte cytotoxic factor (CF) in cytostasis mediated by IFN-gamma-activated monocytes. Immunology. 1985 Oct;56(2):367–372. [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. Mycobacterium lepraemurium infection of nude athymic (nu/nu) mice. Infect Immun. 1985 Jul;49(1):190–196. doi: 10.1128/iai.49.1.190-196.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Masur H., Keithly J. S. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J Immunol. 1982 Jul;129(1):344–350. [PubMed] [Google Scholar]
- Nathan C. F., Prendergast T. J., Wiebe M. E., Stanley E. R., Platzer E., Remold H. G., Welte K., Rubin B. Y., Murray H. W. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984 Aug 1;160(2):600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. A. Trypanosoma cruzi: in vitro induction of macrophage microbicidal activity. J Exp Med. 1978 Jul 1;148(1):288–300. doi: 10.1084/jem.148.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Ellis J., Chaplan S., Cohn Z. Trypanosoma cruzi: in vivo and in vitro correlation between T-cell activation and susceptibility in inbred strains of mice. Exp Parasitol. 1981 Jun;51(3):325–334. doi: 10.1016/0014-4894(81)90120-x. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Kaplan G., Levy E., Sarno E. N., Kushner P., Granelli-Piperno A., Vieira L., Colomer Gould V., Levis W., Steinman R. Defective gamma interferon production in leprosy. Reversal with antigen and interleukin 2. J Exp Med. 1983 Dec 1;158(6):2165–2170. doi: 10.1084/jem.158.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston P. M. Macrophages and protective immunity in Mycobacterium lepraemurium infections in a 'resistant' (C57Bl) and a 'susceptible' (BALB/c) mouse strain. Clin Exp Immunol. 1982 Feb;47(2):243–252. [PMC free article] [PubMed] [Google Scholar]
- Sadick M. D., Locksley R. M., Tubbs C., Raff H. V. Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon-gamma in response to Leishmania antigens in vitro. J Immunol. 1986 Jan;136(2):655–661. [PubMed] [Google Scholar]
- Taylor P. M., Wraith D. C., Askonas B. A. Control of immune interferon release by cytotoxic T-cell clones specific for influenza. Immunology. 1985 Apr;54(4):607–614. [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Kelso A., Louis J. A. Intracellular destruction of Leishmania tropica by macrophages activated with macrophage activating factor/interferon. Clin Exp Immunol. 1984 Jan;55(1):157–165. [PMC free article] [PubMed] [Google Scholar]
- Virelizier J. L. Murine genotype influences the in vitro production of gamma (immune) interferon. Eur J Immunol. 1982 Nov;12(11):988–990. doi: 10.1002/eji.1830121119. [DOI] [PubMed] [Google Scholar]
- Walker L., Lowrie D. B. Killing of Mycobacterium microti by immunologically activated macrophages. Nature. 1981 Sep 3;293(5827):69–71. doi: 10.1038/293069a0. [DOI] [PubMed] [Google Scholar]
- de Gee A. L., Sonnenfeld G., Mansfield J. M. Genetics of resistance to the African trypanosomes. V. Qualitative and quantitative differences in interferon production among susceptible and resistant mouse strains. J Immunol. 1985 Apr;134(4):2723–2726. [PubMed] [Google Scholar]


