Abstract
Immunization of rats with a purified IgE myeloma (IR2) induced an auto-anti-IgE response. Such treatment inhibited total IgE levels in the serum of conventional IgE-producing rats (Marshall & Bell, 1985) and increased the number of mucosal mast cells (MMC) in the intestine. The present study has investigated the ability of auto-anti-IgE induction to influence the course of a Nippostrongylus brasiliensis infection, to modify IgE synthesis, or to affect the number of MMC in the intestine following infection. Auto-anti-IgE induction was found to have a surprising effect on worm elimination. IR2-immunized rats were able to rid themselves of this nematode with an accelerated tempo--a small but significant effect after primary infection, but a substantial enhancement of worm loss after reinfection. Auto-anti-IgE induction was not able to prevent the typical increase in IgE that accompanies an N. brasiliensis infection, nor did it alter the helminth-induced intestinal mastocytosis. When MMC degranulation was measured by assaying the serum levels of a specific rat mast protease (RMCP II) following secondary infection, the amount of RMCP II released was less in auto-anti-IgE-producing rats. These findings have implications for the importance of IgE, MMC and other cells of inflammation in an anti-parasitic response.
Full text
PDF

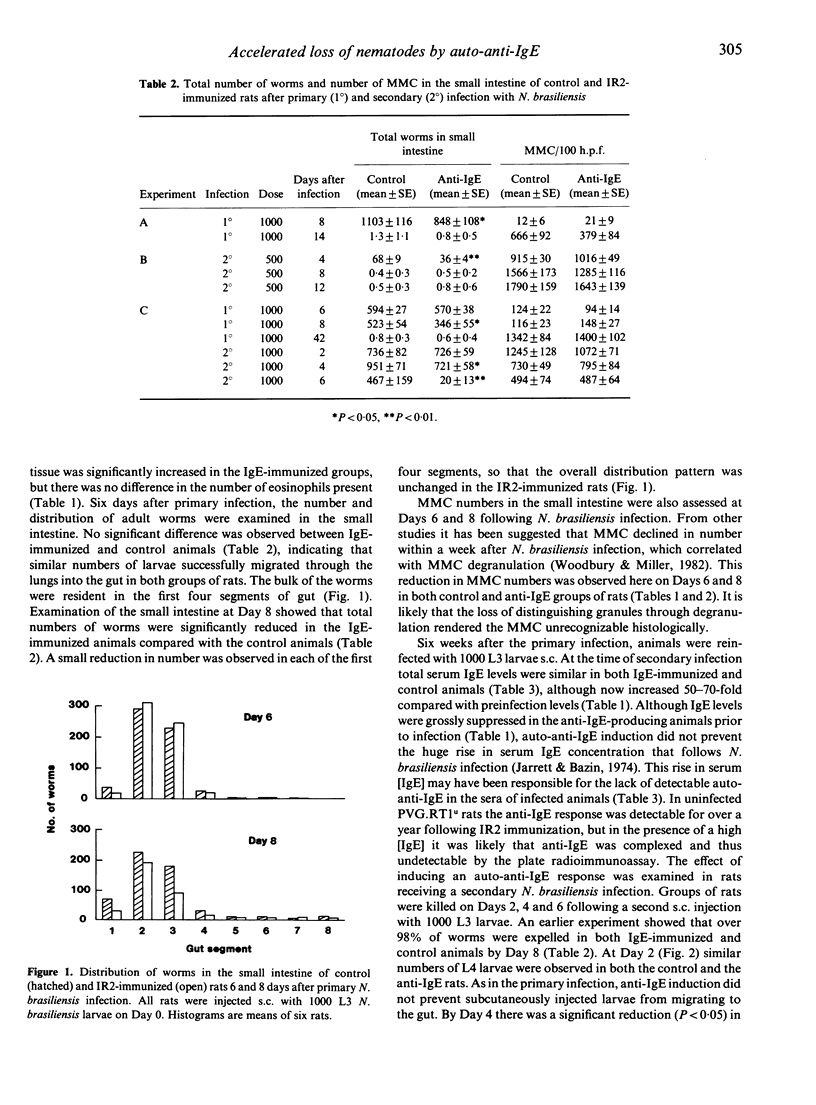
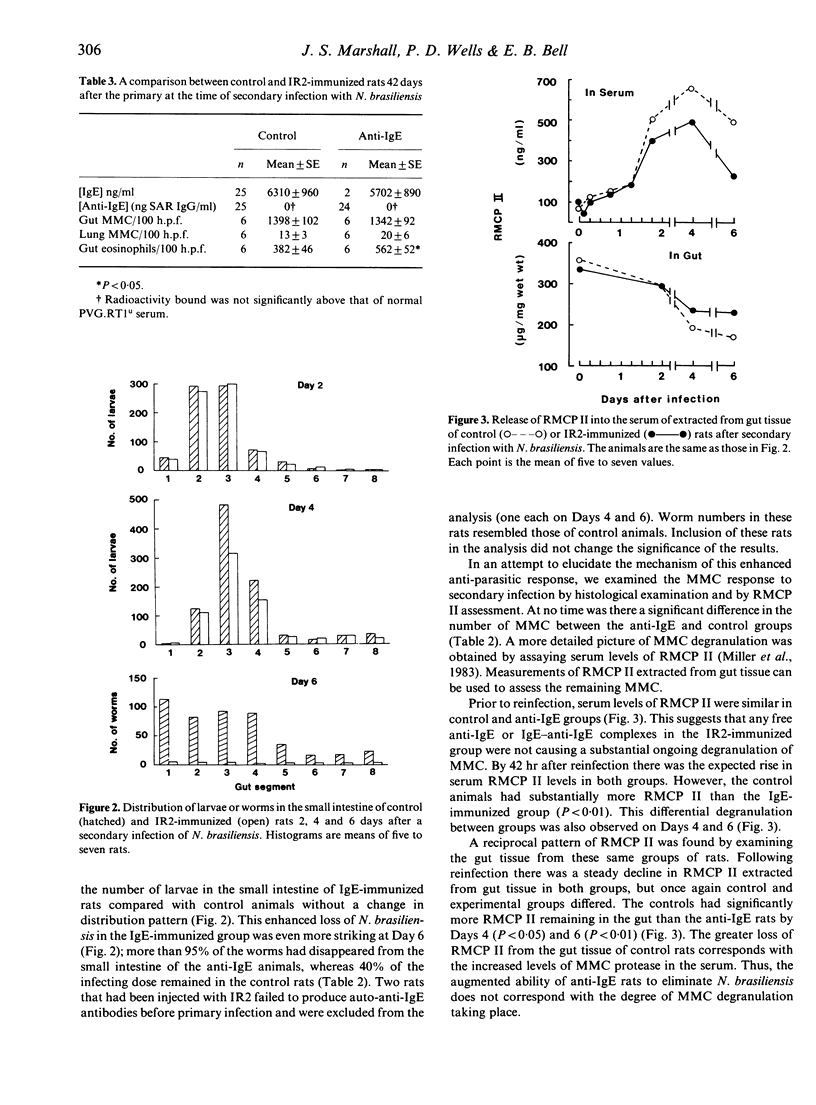


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alphey T. J. Studies on the distribution and site location of Nippostrongylus brasiliensis within the small intestine of laboratory rats. Parasitology. 1970 Dec;61(3):449–460. doi: 10.1017/s0031182000041299. [DOI] [PubMed] [Google Scholar]
- Dessein A. J., Parker W. L., James S. L., David J. R. IgE antibody and resistance to infection. I. Selective suppression of the IgE antibody response in rats diminishes the resistance and the eosinophil response to Trichinella spiralis infection. J Exp Med. 1981 Feb 1;153(2):423–436. doi: 10.1084/jem.153.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. I. Effects of fixation. Acta Pathol Microbiol Scand. 1966;66(3):289–302. doi: 10.1111/apm.1966.66.3.289. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E., Jarrett W. F., Urquhart G. M. Quantitative studies on the kinetics of establishment and expulsion of intestinal nematode populations in susceptible and immune hosts. Nippostrongylus brasiliensis in the rat. Parasitology. 1968 Aug;58(3):625–639. doi: 10.1017/s0031182000028924. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E., Miller H. R. Production and activities of IgE in helminth infection. Prog Allergy. 1982;31:178–233. [PubMed] [Google Scholar]
- Jarrett E., Bazin H. Elevation of total serum IgE in rats following helminth parasite infection. Nature. 1974 Oct 18;251(5476):613–614. doi: 10.1038/251613a0. [DOI] [PubMed] [Google Scholar]
- Karlsson T., Ellerson J. R., Dahlbom I., Bennich H. Analysis of the serum IgE levels in nonimmunized rats of various strains by a radioimmunoassay. Scand J Immunol. 1979 Mar;9(3):217–228. doi: 10.1111/j.1365-3083.1979.tb02725.x. [DOI] [PubMed] [Google Scholar]
- Kay A. B. Eosinophils as effector cells in immunity and hypersensitivity disorders. Clin Exp Immunol. 1985 Oct;62(1):1–12. [PMC free article] [PubMed] [Google Scholar]
- Kelly J. D., Ogilvie B. M. Intestinal mast cell and eosinophil numbers during worm expulsion in nulliparous and lactating rats infected with Nippostrongylus brasiliensis. Int Arch Allergy Appl Immunol. 1972;43(4):497–509. doi: 10.1159/000230865. [DOI] [PubMed] [Google Scholar]
- King S. J., Miller H. R. Anaphylactic release of mucosal mast cell protease and its relationship to gut permeability in Nippostrongylus-primed rats. Immunology. 1984 Apr;51(4):653–660. [PMC free article] [PubMed] [Google Scholar]
- Marshall J. S., Bell E. B. Induction of an auto-anti-IgE response in rats I. Effects on serum IgE concentrations. Eur J Immunol. 1985 Mar;15(3):272–277. doi: 10.1002/eji.1830150312. [DOI] [PubMed] [Google Scholar]
- Miller H. R., Woodbury R. G., Huntley J. F., Newlands G. Systemic release of mucosal mast-cell protease in primed rats challenged with Nippostrongylus brasiliensis. Immunology. 1983 Jul;49(3):471–479. [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg E. J., Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976 Nov 18;264(5583):258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Crocker J. Demonstration of eosinophil polymorph granules in sections of paraffin- and methacrylate-embedded tissue: a new method. Med Lab Sci. 1984 Jul;41(3):288–290. [PubMed] [Google Scholar]
- Strobel S., Miller H. R., Ferguson A. Human intestinal mucosal mast cells: evaluation of fixation and staining techniques. J Clin Pathol. 1981 Aug;34(8):851–858. doi: 10.1136/jcp.34.8.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T. Regulation of reaginic antibody formation in animals. Prog Allergy. 1975;19:122–194. [PubMed] [Google Scholar]
- WELLS P. D. Mast cell, eosinophil and histamine levels in Nippostrongylus brasiliensis infected rats. Exp Parasitol. 1962 Apr;12:82–101. doi: 10.1016/s0014-4894(62)80002-2. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Miller H. R., Huntley J. F., Newlands G. F., Palliser A. C., Wakelin D. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. 1984 Nov 29-Dec 5Nature. 312(5993):450–452. doi: 10.1038/312450a0. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Miller H. R. Quantitative analysis of mucosal mast cell protease in the intestines of Nippostrongylus-infected rats. Immunology. 1982 Jul;46(3):487–495. [PMC free article] [PubMed] [Google Scholar]


