Abstract
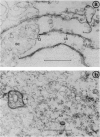
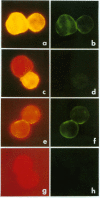
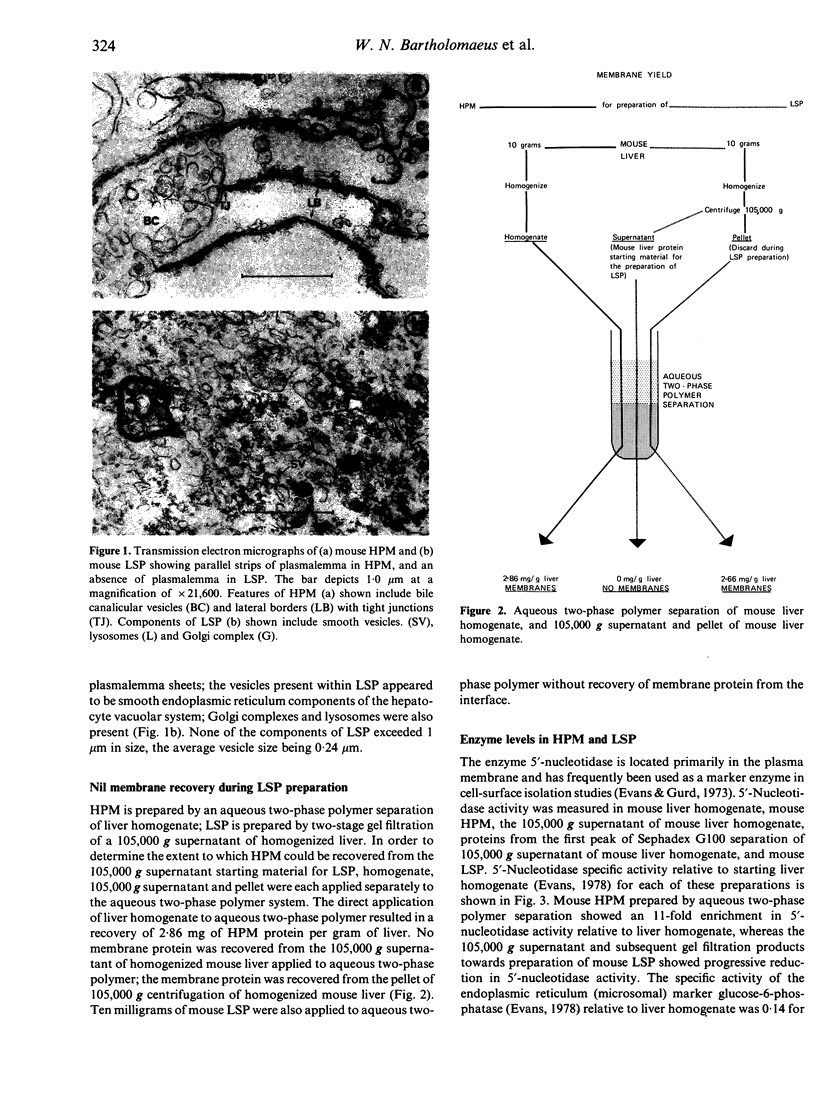
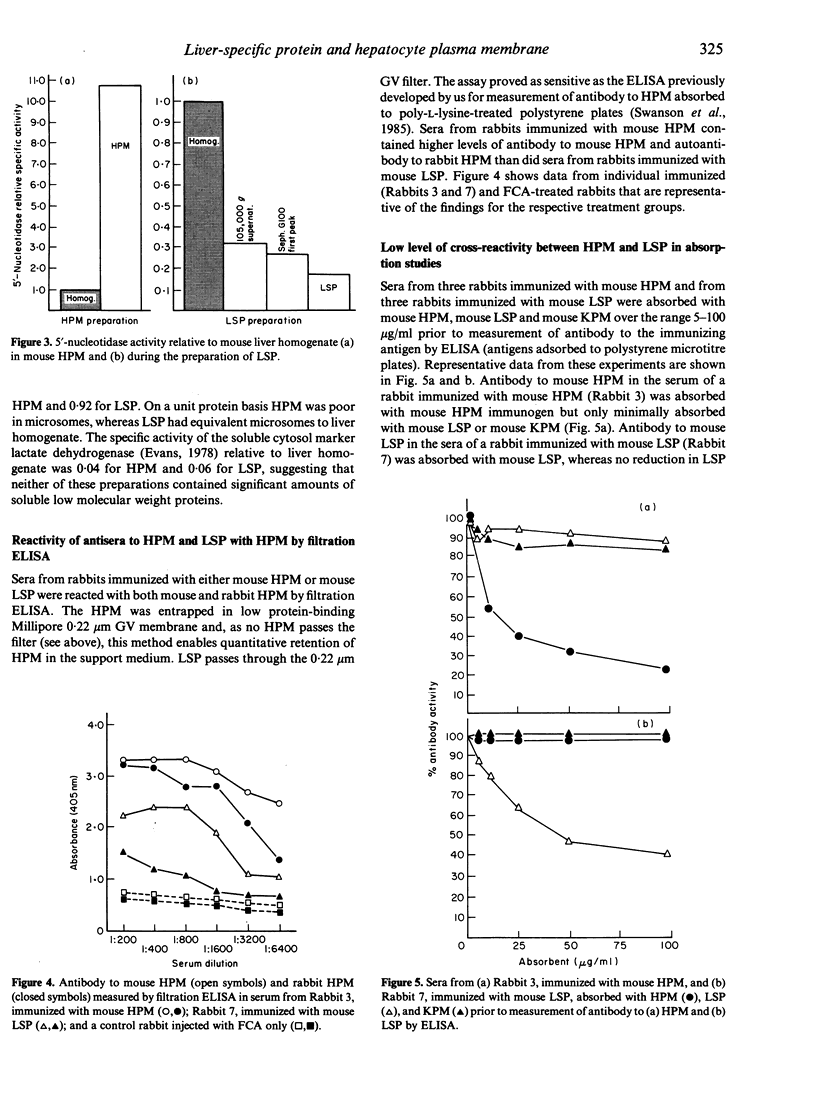
Liver-specific lipoprotein (LSP) has been the subject of intense investigation as a candidate target antigen in chronic active hepatitis. Fundamental to the interest in LSP has been the belief that it is an antigen complex of hepatocyte plasma membrane origin. In this study the physical, biochemical and antigenic relationships between LSP and isolated hepatocyte plasma membrane (HPM) were investigated. Electron microscopic examination of LSP showed it to be devoid of plasmalemma sheets that were abundant in HPM. The plasma membrane marker enzyme 5'-nucleotidase was enriched 11-fold in HPM relative to liver homogenate, whereas the enzyme activity in LSP was 17% of that found in liver homogenate and only 1.5% of that found in HPM. The antigenic relationship between LSP and HPM was assessed using sera from rabbits immunized with either mouse LSP or mouse HPM. By filtration ELISA, antibody to LSP reacted poorly with entrapped HPM, relative to antibody to mouse HPM. Antisera to LSP and HPM were both effectively absorbed by the immunizing antigen, however antibody to LSP was not absorbed with HPM, and minimal cross-absorption of HPM antibody with LSP was found. By immunoblot of SDS-PAGE separated LSP and HPM, it was shown that antigenic cross-reactivity between LSP and HPM at the polypeptide level was rare. By immunofluorescence, antibody to LSP failed to react with the surface of viable mouse hepatocytes, whereas antibody to HPM showed linear fluorescence. The data show that the two preparations, LSP and HPM, are dissimilar antigen complexes. HPM may be a more appropriate preparation for the study of autoimmune liver disease than LSP.
Full text
PDF


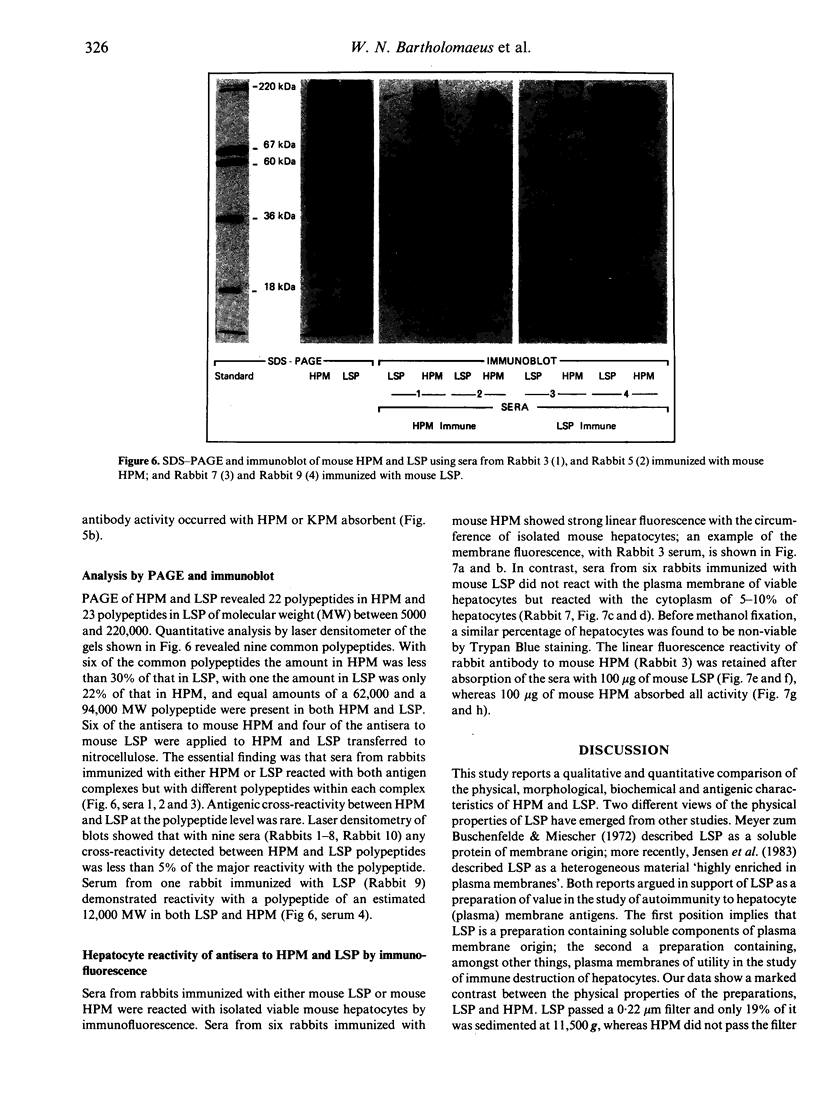
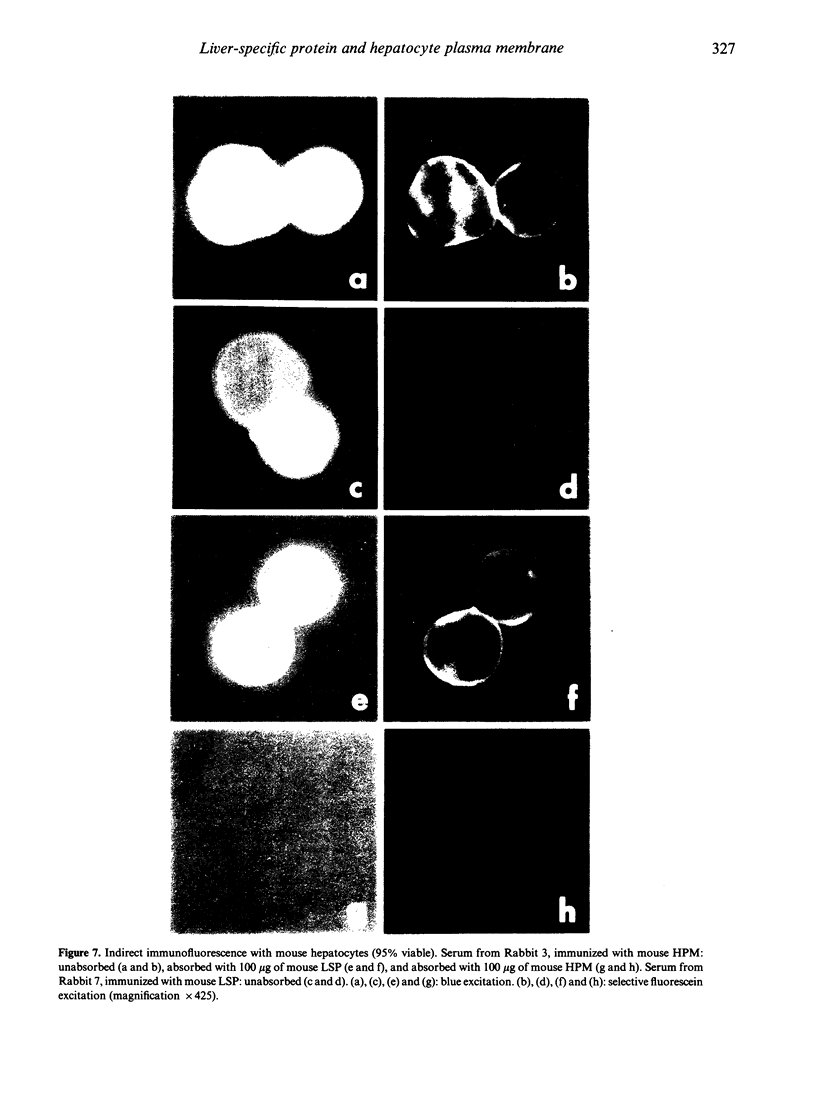


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chisari F. V., Bieber M. S., Josepho C. A., Xavier C., Anderson D. S. Functional properties of lymphocyte subpopulations in hepatitis B virus infection. II. Cytotoxic effector cell killing of targets that naturally express hepatitis B surface antigen and liver-specific lipoprotein. J Immunol. 1981 Jan;126(1):45–49. [PubMed] [Google Scholar]
- De Kretser T. A., McFarlane I. G., Eddleston A. L., Williams R. A species-non-specific liver plasma-membrane antigen and its involvement in chronic active hepatitis. Biochem J. 1980 Mar 15;186(3):679–685. doi: 10.1042/bj1860679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Gurd J. W. Properties of a 5'-nucleotidase purified from mouse liver plasma membranes. Biochem J. 1973 May;133(1):189–199. doi: 10.1042/bj1330189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighery C., Weir D. G. How specific is liver-specific protein? Gastroenterology. 1980 Jul;79(1):179–180. [PubMed] [Google Scholar]
- Glossmann H., Gips H. The preparation of brush border membranes from rat kidney using an aqueous two-phase polymer system. Naunyn Schmiedebergs Arch Pharmacol. 1974;282(4):439–444. doi: 10.1007/BF00500992. [DOI] [PubMed] [Google Scholar]
- Gurd J. W., Evans W. H., Perkins H. R. Immunochemical characterization of proteins from mouse liver plasma membranes. Biochem J. 1973 Dec;135(4):827–832. doi: 10.1042/bj1350827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurd J. W., Evans W. H., Perkins H. R. The distribution of surface antigens during fractionation of mouse liver plasma membranes. Biochem J. 1972 Nov;130(1):271–280. doi: 10.1042/bj1300271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf U., Meyer zum Büschenfelde K. H., Freudenberg J. Liver-specific antigens of different species. II. Localization of a membrane antigen at cell surface of isolated hepatocytes. Clin Exp Immunol. 1974 Jan;16(1):117–123. [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Wall D. A., Ma A. Isolation of rat hepatocyte plasma membranes. I. Presence of the three major domains. J Cell Biol. 1983 Jan;96(1):217–229. doi: 10.1083/jcb.96.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen D. M., Hall C., Majewski T. The plasma membrane origin of liver-specific protein (LSP). Liver. 1983 Aug;3(4):213–219. doi: 10.1111/j.1600-0676.1983.tb00870.x. [DOI] [PubMed] [Google Scholar]
- Korzeniewski C., Callewaert D. M. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983 Nov 25;64(3):313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- Lahav M., Schoenfeld N., Epstein O., Atsmon A. A method for obtaining high recovery of purified subcellular fractions of rat liver homogenate. Anal Biochem. 1982 Mar 15;121(1):114–122. doi: 10.1016/0003-2697(82)90563-2. [DOI] [PubMed] [Google Scholar]
- Lebwohl N., Gerber M. A. Characterization and demonstration of human liver-specific protein (LSP) and apo-LSP. Clin Exp Immunol. 1981 Nov;46(2):435–442. [PMC free article] [PubMed] [Google Scholar]
- Lesko L., Donlon M., Marinetti G. V., Hare J. D. A rapid method for the isolation of rat liver plasma membranes using an aqueous two-phase polymer system. Biochim Biophys Acta. 1973 Jun 22;311(2):173–179. doi: 10.1016/0005-2736(73)90264-2. [DOI] [PubMed] [Google Scholar]
- McFarlane I. G. Autoimmunity in liver disease. Clin Sci (Lond) 1984 Dec;67(6):569–578. doi: 10.1042/cs0670569. [DOI] [PubMed] [Google Scholar]
- McFarlane I. G., Wojcicka B. M., Williams R. Antigens of the human liver. Clin Exp Immunol. 1980 Apr;40(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- McFarlane I. G., Wojcicka B. M., Zucker G. M., Eddleston A. L., Williams R. Purification and characterization of human liver-specific membrane lipoprotein (LSP). Clin Exp Immunol. 1977 Mar;27(3):381–390. [PMC free article] [PubMed] [Google Scholar]
- Meyer zum Büschenfelde K. H., Manns M., Hütteroth T. H., Hopf U., Arnold W. LM-Ag and LSP--two different target antigens involved in the immunopathogenesis of chronic active hepatitis? Clin Exp Immunol. 1979 Aug;37(2):205–212. [PMC free article] [PubMed] [Google Scholar]
- Pertoft H., Rubin K., Kjellén L., Laurent T. C., Klingeborn B. The viability of cells grown or centrifuged in a new density gradient medium, Percoll(TM). Exp Cell Res. 1977 Dec;110(2):449–457. doi: 10.1016/0014-4827(77)90311-1. [DOI] [PubMed] [Google Scholar]
- Pesce M. A., Strande C. S. A new micromethod for determination of protein in cerebrospinal fluid and urine. Clin Chem. 1973 Nov;19(11):1265–1267. [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. I. Effect of Ca 2+ on enzymatic dispersion of isolated, perfused liver. Exp Cell Res. 1972 Oct;74(2):450–454. doi: 10.1016/0014-4827(72)90400-4. [DOI] [PubMed] [Google Scholar]
- Swanson N. R., Bartholomaeus W. N., Reed W. D., Joske R. A. An enzyme-linked immunosorbent assay for the detection of hepatocyte plasma membrane antibodies. J Immunol Methods. 1985 Dec 17;85(1):203–216. doi: 10.1016/0022-1759(85)90288-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]