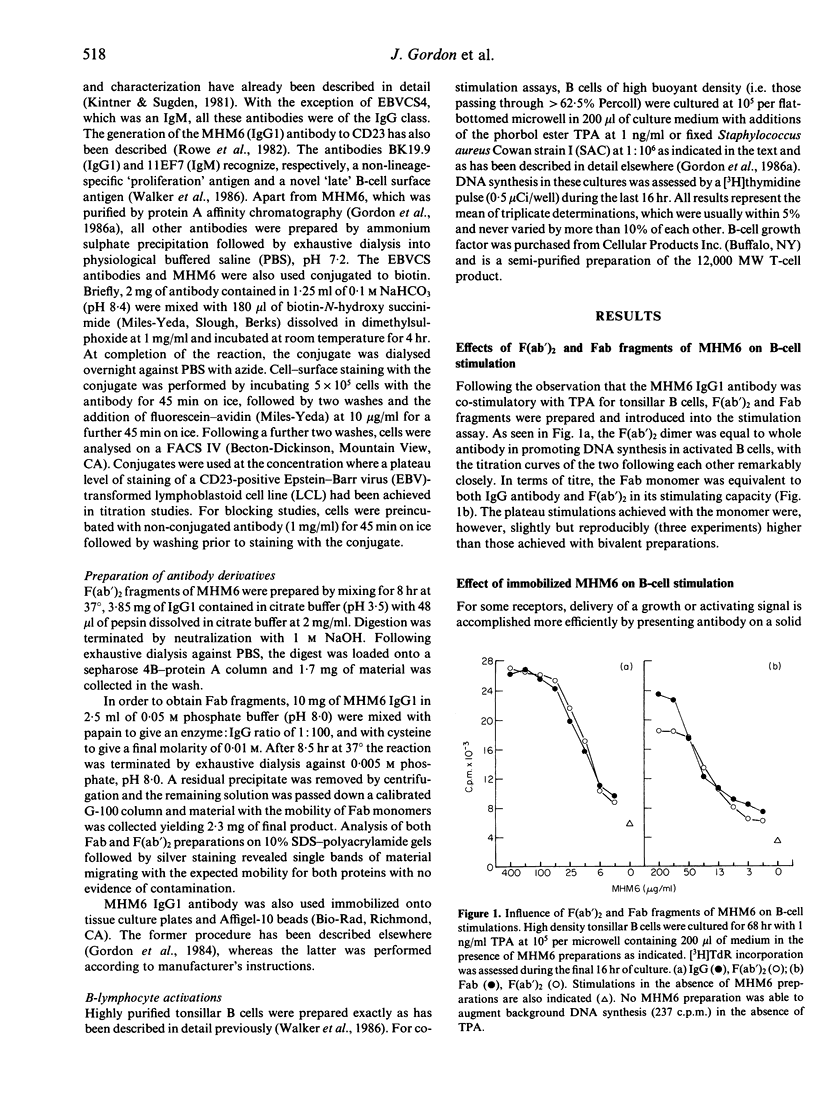
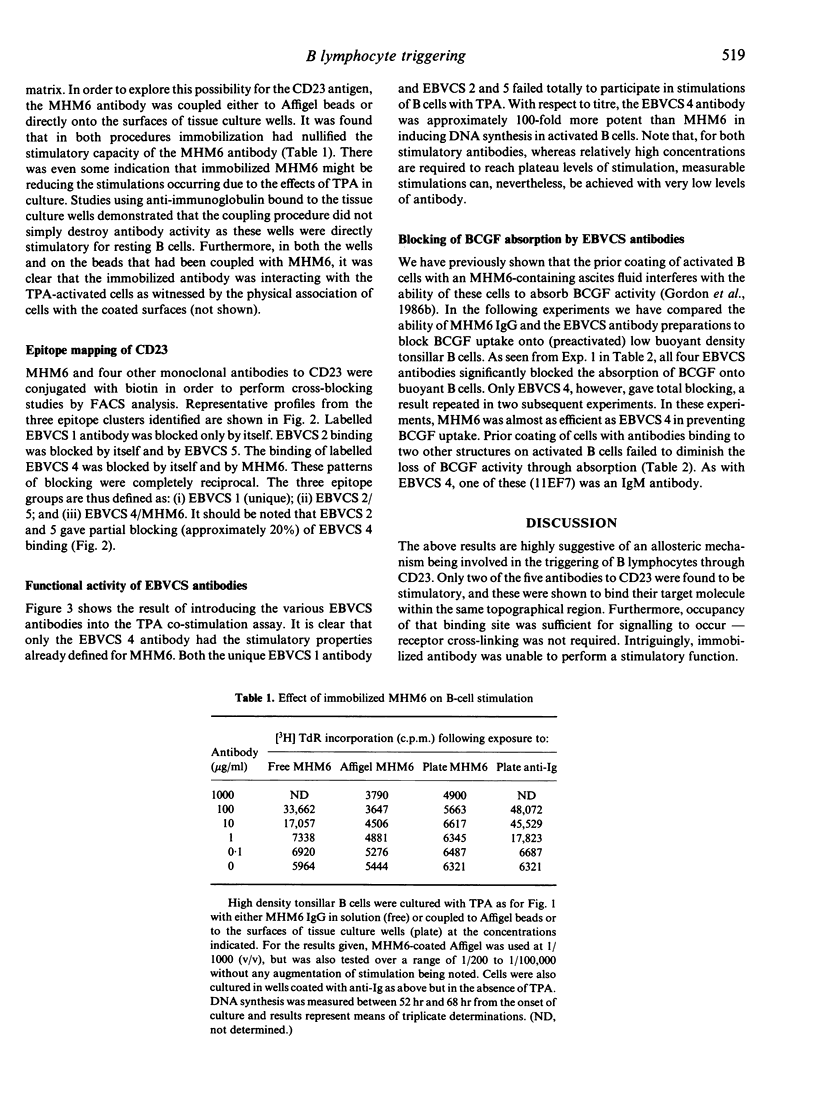
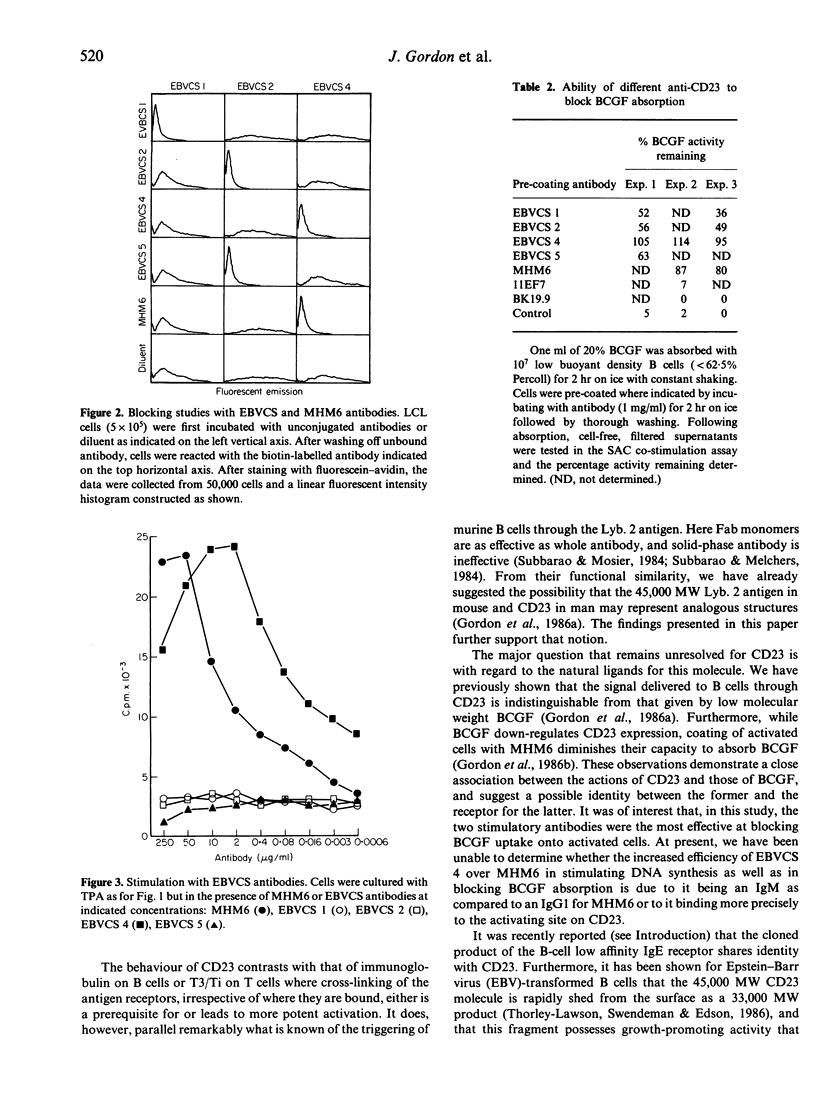
Abstract
By using five monoclonal antibodies in reciprocal cross-locking studies, a minimum of three epitope clusters have been defined for the B-cell restricted, activation-associated CD23 antigen. Two of the five antibodies were capable of replacing low molecular weight B-cell growth factor (BCGF) in B-cell co-stimulation assays. These two antibodies belonged to the same epitope group, while non-stimulatory antibodies fell outside this cluster. By prior coating of activated B lymphocytes at 4 degrees, all five CD23 antibodies interfered with the subsequent uptake of BCGF activity onto the cells. However, only the two stimulatory antibodies were capable of inhibiting the absorption of BCGF completely. From one of these antibodies, F(ab')2 and Fab fragments were generated and both were found to be equivalent to whole antibody in their ability to mimic BCGF. Immobilized antibody, however, failed to stimulate over a wide range of concentrations. These findings demonstrate that the ability of certain CD23 antibodies to deliver a growth-promoting signal to activated B cells is independent not only of the Fc portion of the molecule but also of receptor cross-linking. The latter observation is indicative of an allosteric mechanism of triggering, a notion supported by the epitope specificity of activation through CD23. The findings are discussed in relation to the putative natural ligands for CD23 and the way they may influence B-cell function through this receptor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark E. A., Ledbetter J. A. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4494–4498. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörken B., Moldenhauer G., Pezzutto A., Schwartz R., Feller A., Kiesel S., Nadler L. M. HD39 (B3), a B lineage-restricted antigen whose cell surface expression is limited to resting and activated human B lymphocytes. J Immunol. 1986 Jun 15;136(12):4470–4479. [PubMed] [Google Scholar]
- Gordon J., Ley S. C., Melamed M. D., English L. S., Hughes-Jones N. C. Immortalized B lymphocytes produce B-cell growth factor. Nature. 1984 Jul 12;310(5973):145–147. doi: 10.1038/310145a0. [DOI] [PubMed] [Google Scholar]
- Gordon J., Rowe M., Walker L., Guy G. Ligation of the CD23,p45 (BLAST-2,EBVCS) antigen triggers the cell-cycle progression of activated B lymphocytes. Eur J Immunol. 1986 Sep;16(9):1075–1080. doi: 10.1002/eji.1830160908. [DOI] [PubMed] [Google Scholar]
- Gordon J., Webb A. J., Walker L., Guy G. R., Rowe M. Evidence for an association between CD23 and the receptor for a low molecular weight B cell growth factor. Eur J Immunol. 1986 Dec;16(12):1627–1630. doi: 10.1002/eji.1830161225. [DOI] [PubMed] [Google Scholar]
- Kikutani H., Inui S., Sato R., Barsumian E. L., Owaki H., Yamasaki K., Kaisho T., Uchibayashi N., Hardy R. R., Hirano T. Molecular structure of human lymphocyte receptor for immunoglobulin E. Cell. 1986 Dec 5;47(5):657–665. doi: 10.1016/0092-8674(86)90508-8. [DOI] [PubMed] [Google Scholar]
- Kintner C., Sugden B. Identification of antigenic determinants unique to the surfaces of cells transformed by Epstein-Barr virus. Nature. 1981 Dec 3;294(5840):458–460. doi: 10.1038/294458a0. [DOI] [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Pezzutto A., Dörken B., Moldenhauer G., Clark E. A. Amplification of human B cell activation by a monoclonal antibody to the B cell-specific antigen CD22, Bp 130/140. J Immunol. 1987 Jan 1;138(1):98–103. [PubMed] [Google Scholar]
- Rowe M., Hildreth J. E., Rickinson A. B., Epstein M. A. Monoclonal antibodies to Epstein-Barr virus-induced, transformation-associated cell surface antigens: binding patterns and effect upon virus-specific T-cell cytotoxicity. Int J Cancer. 1982 Apr 15;29(4):373–381. doi: 10.1002/ijc.2910290403. [DOI] [PubMed] [Google Scholar]
- Subbarao B., Melchers F. The action of an Lyb2.1-specific monoclonal antibody in soluble or immobilized form on resting and activated B cells. Curr Top Microbiol Immunol. 1984;113:72–76. doi: 10.1007/978-3-642-69860-6_14. [DOI] [PubMed] [Google Scholar]
- Subbarao B., Mosier D. E. Activation of B lymphocytes by monovalent anti-Lyb-2 antibodies. J Exp Med. 1984 Jun 1;159(6):1796–1801. doi: 10.1084/jem.159.6.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suemura M., Kikutani H., Barsumian E. L., Hattori Y., Kishimoto S., Sato R., Maeda A., Nakamura H., Owaki H., Hardy R. R. Monoclonal anti-Fc epsilon receptor antibodies with different specificities and studies on the expression of Fc epsilon receptors on human B and T cells. J Immunol. 1986 Aug 15;137(4):1214–1220. [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Swendeman S. L., Edson C. M. Biochemical analysis suggests distinct functional roles for the BLAST-1 and BLAST-2 antigens. J Immunol. 1986 Mar 1;136(5):1745–1751. [PubMed] [Google Scholar]
- Walker L., Guy G., Brown G., Rowe M., Milner A. E., Gordon J. Control of human B-lymphocyte replication. I. Characterization of novel activation states that precede the entry of G0 B cells into cycle. Immunology. 1986 Aug;58(4):583–589. [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Otsuka T., Mosmann T., Banchereau J., DeFrance T., Blanchard D., De Vries J. E., Lee F., Arai K. Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5894–5898. doi: 10.1073/pnas.83.16.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]


