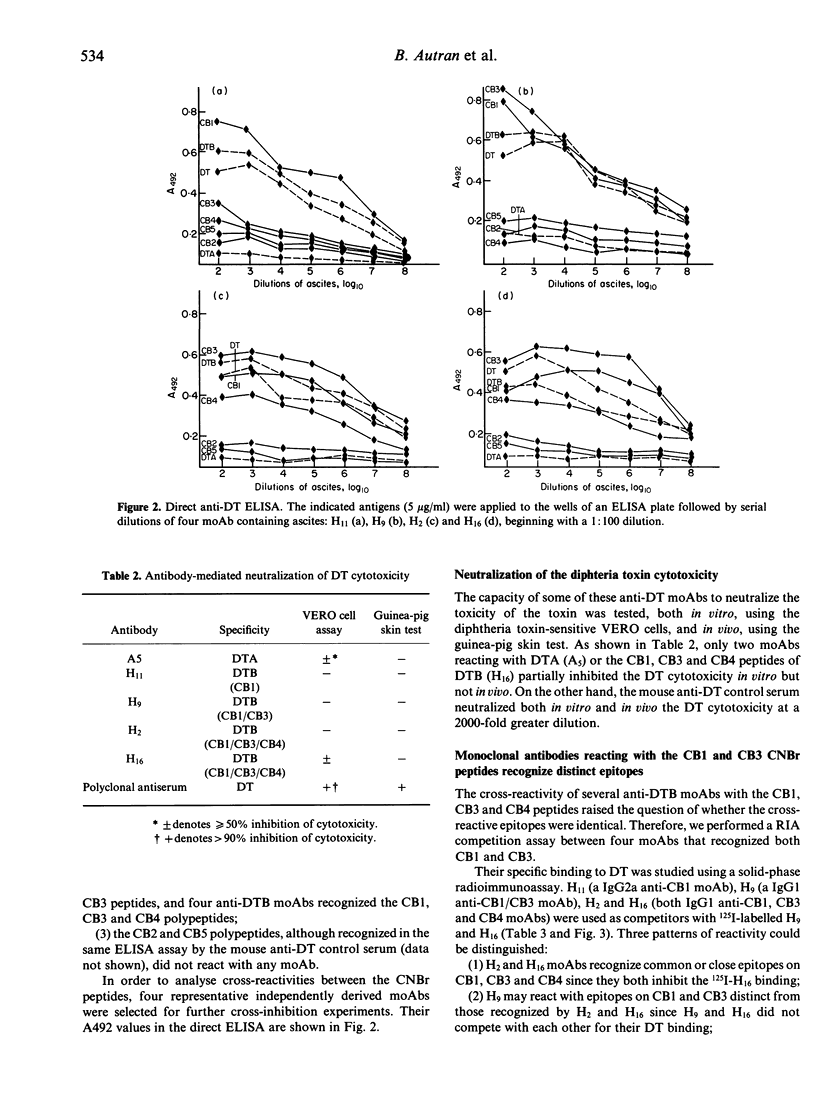
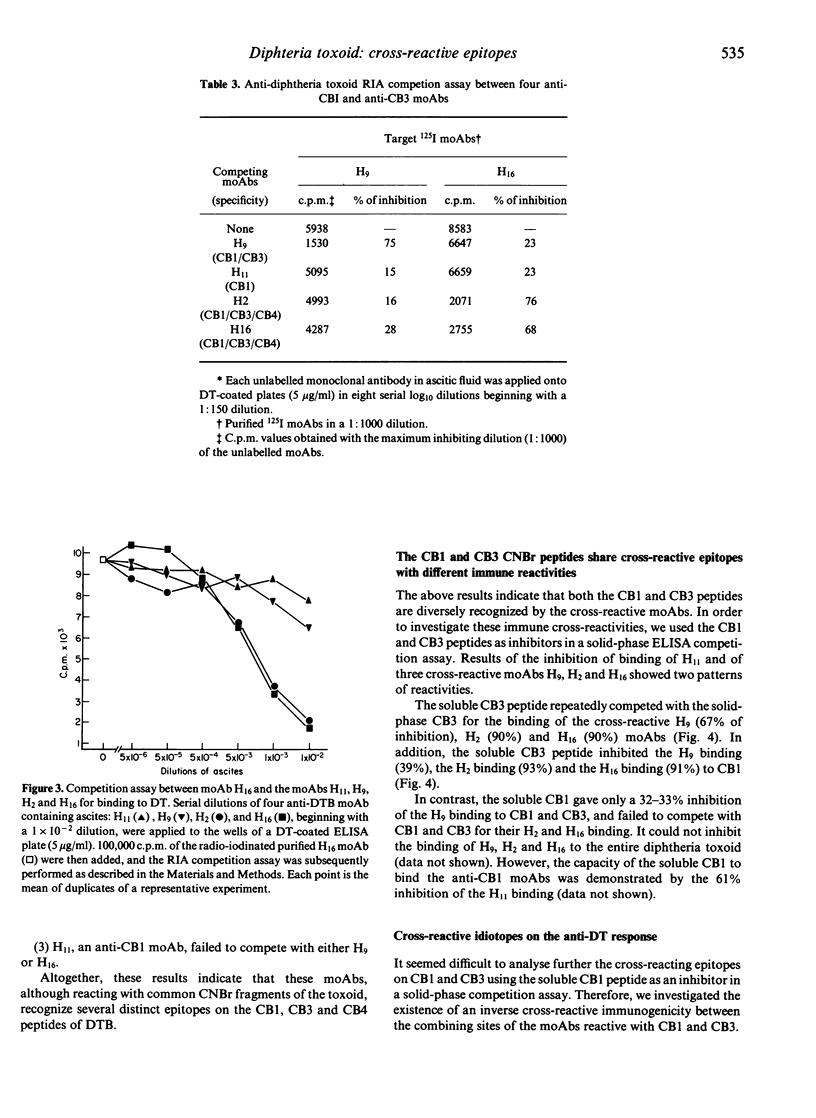
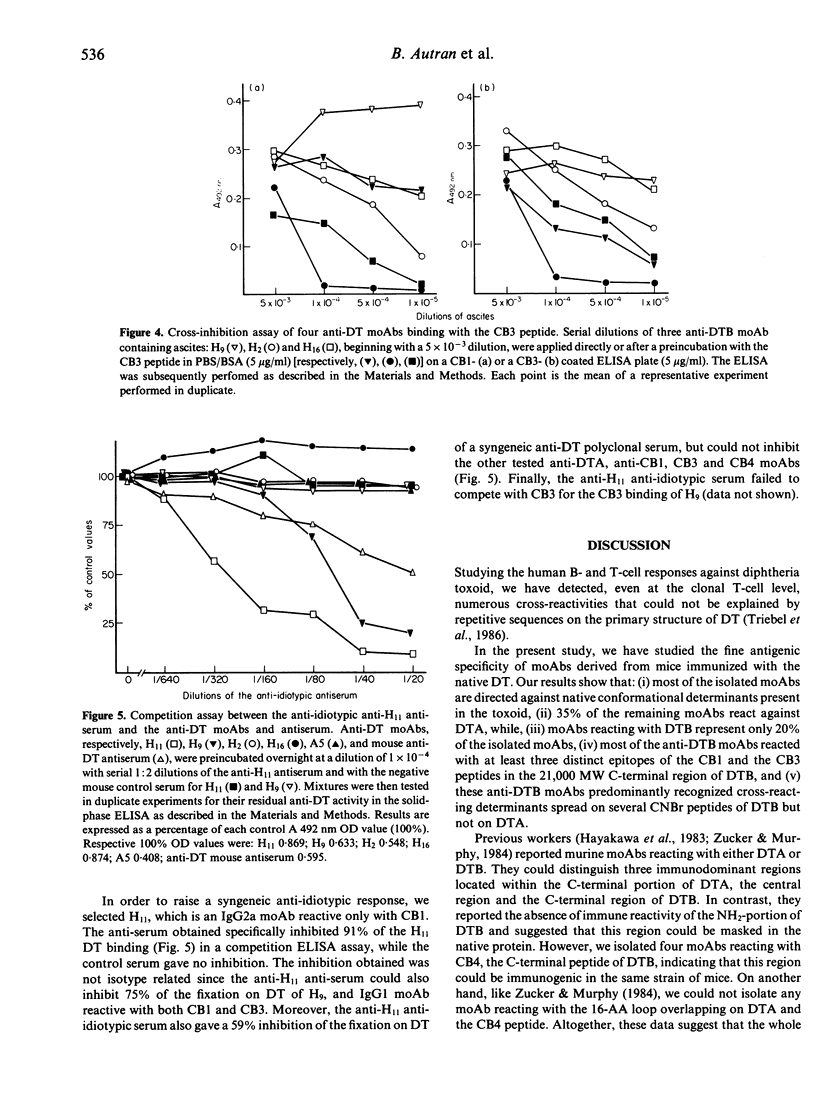
Abstract
Our previous studies on the human specific B- and T-cell responses against diphtheria toxoid (DT) had shown that the B- and T-cell antigenic determinants were mostly assembled topographic sites with large intra-molecular cross-reactivities. In order to characterize further the B-cell responses against DT, and to investigate these cross-reactivities, we have produced 54 murine monoclonal antibodies (moAbs) against the native DT. Determination of their antigenic specificity showed that 25 moAbs recognized conformational determinants on the native DT, 20 moAbs reacted against the A fragment (DTA) of the toxin, and nine moAbs reacted with at least three distinct highly purified CNBr peptides of the B fragment (DTB); some of them simultaneously recognized two or three purified CNBr peptides of DTB. As shown by cross-inhibition studies, the epitopes cross-reacting with those moAbs were distinct. Previously described sequence data indicated that these epitopes were not induced by repetitive amino-acid sequences. Finally, a cross-reactive idiotype was shared by moAbs specific for the CB1 and the CB3 peptides of DTB. Altogether, these data indicate that anti-DT moAbs are mostly directed against conformational determinants, and may cross-react with several DTB CNBr peptides.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audibert F., Jolivet M., Chedid L., Alouf J. E., Boquet P., Rivaille P., Siffert O. Active antitoxic immunization by a diphtheria toxin synthetic oligopeptide. Nature. 1981 Feb 12;289(5798):593–594. doi: 10.1038/289593a0. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Goscienski P. J., Hamburger R. N. Characteristics of human antibody to diphtheria toxin. Infect Immun. 1973 Feb;7(2):130–136. doi: 10.1128/iai.7.2.130-136.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Benjamini E., Shimizu M., Young J. D., Leung C. Y. Immunochemical studies on the tobacco mosaic virus protein. VII. The binding of octanoylated peptides of the tobacco mosaic virus protein with antibodies to the whole protein. Biochemistry. 1968 Apr;7(4):1261–1264. doi: 10.1021/bi00844a002. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A., Buckenmeyer G. K., Hicks G., Gurd F. R., Feldmann R. J., Minna J. Topographic antigenic determinants recognized by monoclonal antibodies to sperm whale myoglobin. J Biol Chem. 1982 Mar 25;257(6):3189–3198. [PubMed] [Google Scholar]
- Berzofsky J. A. Intrinsic and extrinsic factors in protein antigenic structure. Science. 1985 Sep 6;229(4717):932–940. doi: 10.1126/science.2410982. [DOI] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Cryz S. J., Welkos S. L., Holmes R. K. Immunochemical studies of diphtherial toxin and related nontoxic mutant proteins. Infect Immun. 1980 Dec;30(3):835–846. doi: 10.1128/iai.30.3.835-846.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C., Labit C., Marchetto S., Pierres M. Shared idiotope on monoclonal anti-Ia.7 antibodies reactive with determinants in a structural domain of the I-E molecules. J Immunol. 1984 Mar;132(3):1353–1360. [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr Structure-activity relationships in diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1492–1495. [PubMed] [Google Scholar]
- Greenfield L., Bjorn M. J., Horn G., Fong D., Buck G. A., Collier R. J., Kaplan D. A. Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage beta. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6853–6857. doi: 10.1073/pnas.80.22.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa S., Uchida T., Mekada E., Moynihan M. R., Okada Y. Monoclonal antibody against diphtheria toxin. Effect on toxin binding and entry into cells. J Biol Chem. 1983 Apr 10;258(7):4311–4317. [PubMed] [Google Scholar]
- Hiernaux J., Bona C. A. Shared idiotypes among monoclonal antibodies specific for different immunodominant sugars of lipopolysaccharide of different Gram-negative bacteria. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1616–1620. doi: 10.1073/pnas.79.5.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C. O., Sela M., Arnon R. Antibodies against synthetic peptides of the B subunit of cholera toxin: crossreaction and neutralization of the toxin. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7611–7615. doi: 10.1073/pnas.80.24.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorek M., Delpeyroux F., Chenciner N., Streeck R. E., Murphy J. R., Boquet P., Tiollais P. Nucleotide sequence and expression of the diphtheria tox228 gene in Escherichia coli. Science. 1983 Aug 26;221(4613):855–858. doi: 10.1126/science.6348945. [DOI] [PubMed] [Google Scholar]
- Kohno Y., Berkower I., Minna J., Berzofsky J. A. Idiotypes of anti-myoglobin antibodies: shared idiotypes among monoclonal antibodies to distinct determinants of sperm whale myoglobin. J Immunol. 1982 Apr;128(4):1742–1748. [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Sheppard A. J., Cussell D., Hughes M. Production and characterization of monoclonal antibodies to tetanus toxin. Infect Immun. 1984 Feb;43(2):710–714. doi: 10.1128/iai.43.2.710-714.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P. T., Riehm J. P., Todd P. E., Leach S. J. The antigenicity of myoglobin-related peptides synthesised on polyacrylamide and polystyrene resin supports. Mol Immunol. 1984 Jun;21(6):489–496. doi: 10.1016/0161-5890(84)90064-6. [DOI] [PubMed] [Google Scholar]
- Smith-Gill S. J., Mainhart C. R., Lavoie T. B., Rudikoff S., Potter M. VL-VH expression by monoclonal antibodies recognizing avian lysozyme. J Immunol. 1984 Feb;132(2):963–967. [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Triebel F., Autran B., De Roquefeuil S., Falmagne P., Debré P. Immune response to diphtheria toxin and to different CNBr fragments: evidence for different B and T cell reactivities. Eur J Immunol. 1986 Jan;16(1):47–53. doi: 10.1002/eji.1830160110. [DOI] [PubMed] [Google Scholar]
- Zucker D. R., Murphy J. R. Monoclonal antibody analysis of diphtheria toxin--I. Localization of epitopes and neutralization of cytotoxicity. Mol Immunol. 1984 Sep;21(9):785–793. doi: 10.1016/0161-5890(84)90165-2. [DOI] [PubMed] [Google Scholar]


