Abstract
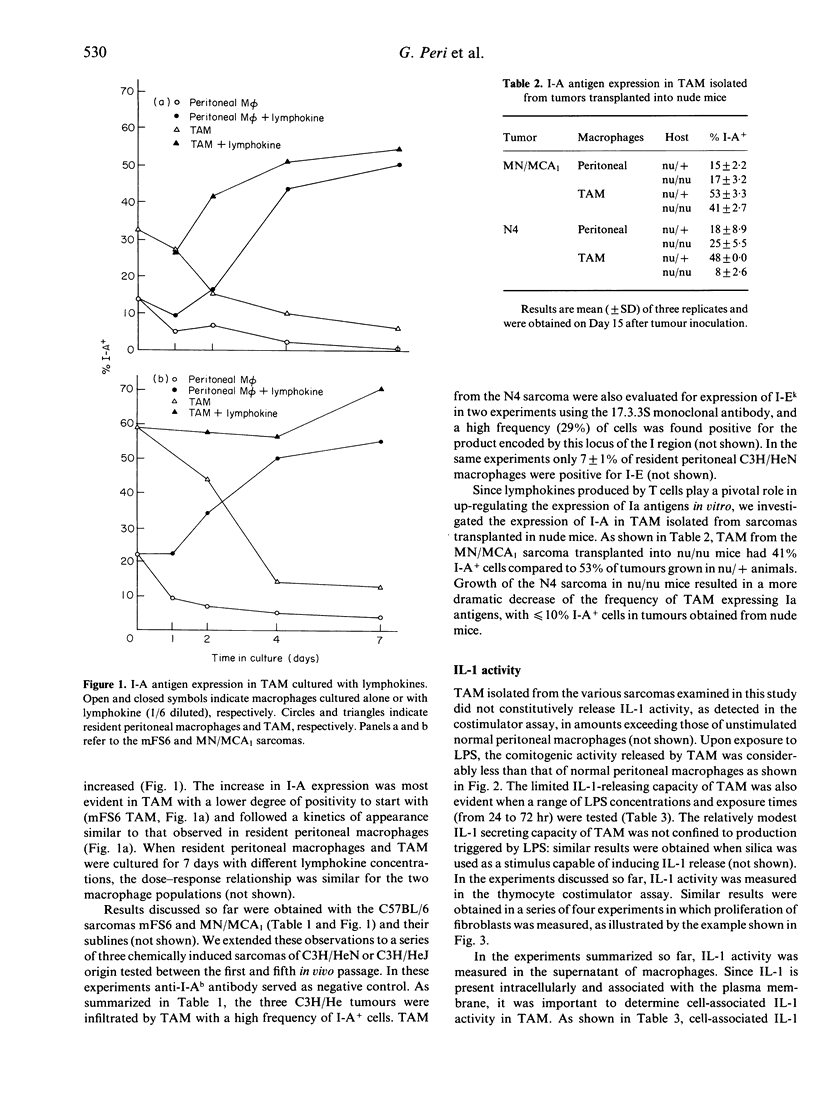
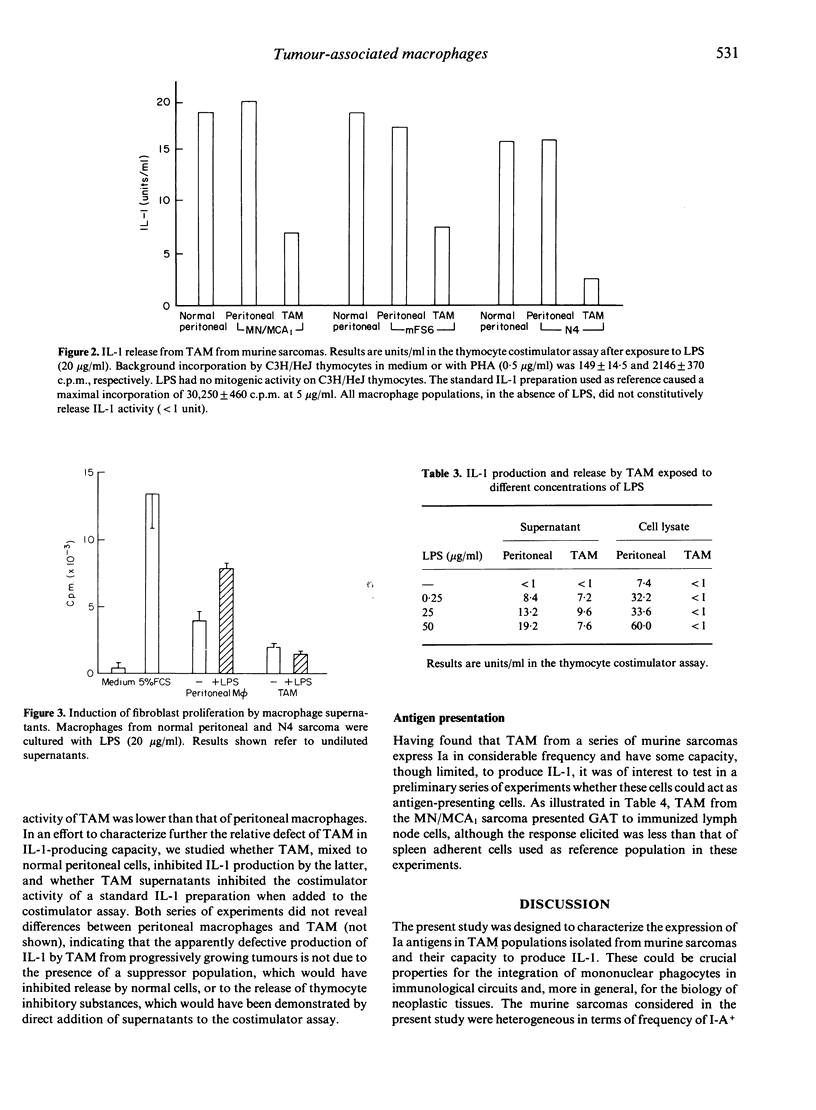
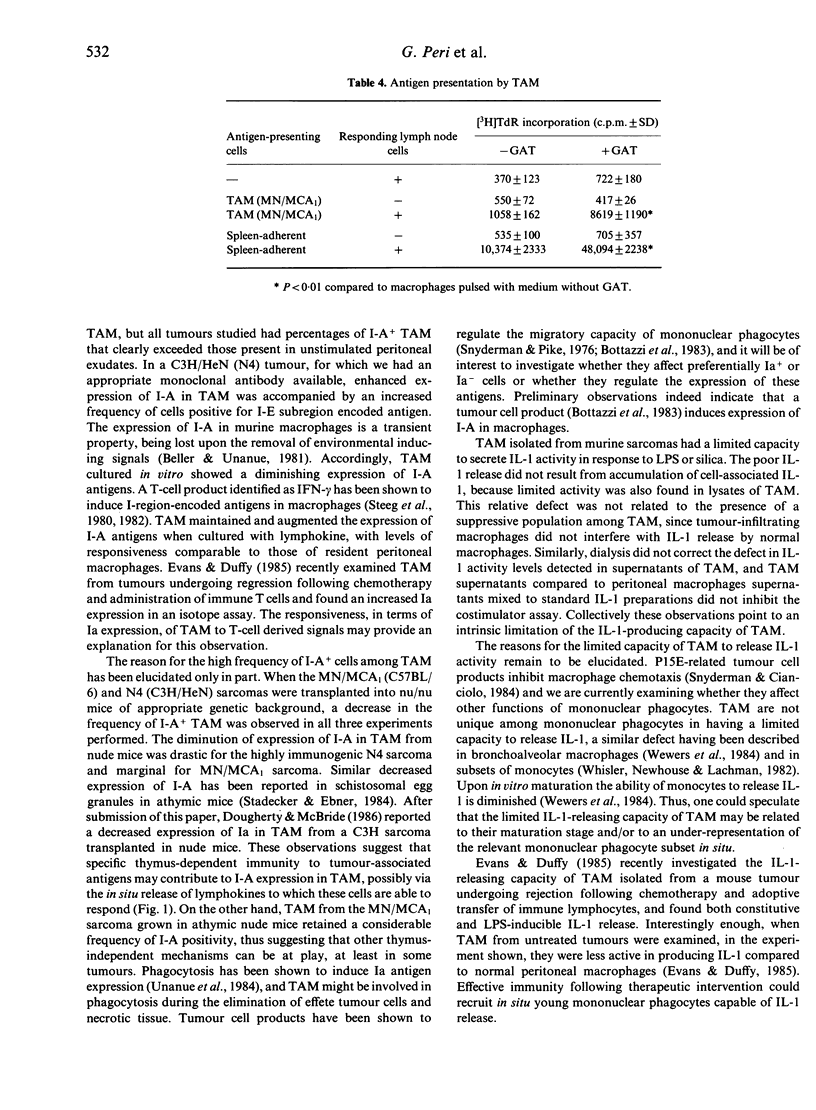
Tumour-associated macrophages (TAM) isolated from five murine sarcomas had a relatively high frequency of I-A+ cells, with mean values of 27% (mFS6), 52% (MN/MCA1), 68% (N3), 62% (N4) and 98% (J3) for TAM compared to 12% for resident peritoneal macrophages. Expression of I-E in TAM was also high (29%) in the only sarcoma (N4) examined in this respect. Expression of I-A by TAM declined in culture but exposure to lymphokine supernatants maintained and increased the frequency of I-A+ cells in TAM. Transplantation of tumours into nude mice caused a marked decrease in the percentage of I-A+ TAM in the case of the N4 sarcoma (8% compared to 48%), whereas for the MN/MCA1 sarcoma the diminution was only marginal (from 53 to 41%), TAM from murine sarcomas did not constitutively release appreciable levels of interleukin-1 (IL-1) activity. Upon stimulation with bacterial lipopolysaccharides or silica, TAM showed a limited capacity to produce and release IL-1 activity compared to peritoneal macrophages. Thus the expression of I-A antigens and the IL-1-producing capacity are uncoupled in TAM from murine sarcomas. These properties of TAM could play an important role in the generation of anti-tumour immunity and/or of suppressive T-cell circuits.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beller D. I., Unanue E. R. Regulation of macrophage populations. II. Synthesis and expression of Ia antigens by peritoneal exudate macrophages is a transient event. J Immunol. 1981 Jan;126(1):263–269. [PubMed] [Google Scholar]
- Bottazzi B., Polentarutti N., Acero R., Balsari A., Boraschi D., Ghezzi P., Salmona M., Mantovani A. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983 Apr 8;220(4593):210–212. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- Dougherty G. J., McBride W. H. Accessory cell activity of murine tumor-associated macrophages. J Natl Cancer Inst. 1986 Mar;76(3):541–548. [PubMed] [Google Scholar]
- Eccles S. A., Alexander P. Macrophage content of tumours in relation to metastatic spread and host immune reaction. Nature. 1974 Aug 23;250(5468):667–669. doi: 10.1038/250667a0. [DOI] [PubMed] [Google Scholar]
- Evans R., Duffy T. M. Amplification of immune T lymphocyte function in situ: the identification of active components of the immunologic network during tumor rejection. J Immunol. 1985 Aug;135(2):1498–1504. [PubMed] [Google Scholar]
- Evans R., Eidlen D. M. Macrophage accumulation in transplanted tumors is not dependent on host immune responsiveness or presence of tumor-associated rejection antigens. J Reticuloendothel Soc. 1981 Nov;30(5):425–437. [PubMed] [Google Scholar]
- Evans R. Macrophages in syngeneic animal tumours. Transplantation. 1972 Oct;14(4):468–473. doi: 10.1097/00007890-197210000-00011. [DOI] [PubMed] [Google Scholar]
- Gery I., Davies P., Derr J., Krett N., Barranger J. A. Relationship between production and release of lymphocyte-activating factor (interleukin 1) by murine macrophages. 1. Effects of various agents. Cell Immunol. 1981 Nov 1;64(2):293–303. doi: 10.1016/0008-8749(81)90481-0. [DOI] [PubMed] [Google Scholar]
- Kaizer L., Lala P. K. Post-mitotic age of monocuclear cells migrating into TA-3(St) solid tumors. Cell Tissue Kinet. 1977 May;10(3):279–288. doi: 10.1111/j.1365-2184.1977.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Mantovani A. Effects on in vitro tumor growth of murine macrophages isolated from sarcoma lines differing in immunogenicity and metastasizing capacity. Int J Cancer. 1978 Dec;22(6):741–746. doi: 10.1002/ijc.2910220617. [DOI] [PubMed] [Google Scholar]
- Mantovani A. In vitro effects on tumor cells of macrophages isolated from an early-passage chemically-induced murine sarcoma and from its spontaneous metastases. Int J Cancer. 1981 Feb 15;27(2):221–228. doi: 10.1002/ijc.2910270215. [DOI] [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Unanue E. R., Cotran R. S. Stimulation of nonlymphoid mesenchymal cell proliferation by a macrophage-derived growth factor. J Immunol. 1981 Apr;126(4):1510–1515. [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Rossi V., Breviario F., Ghezzi P., Dejana E., Mantovani A. Prostacyclin synthesis induced in vascular cells by interleukin-1. Science. 1985 Jul 12;229(4709):174–176. doi: 10.1126/science.2409598. [DOI] [PubMed] [Google Scholar]
- Schook L. B., Allen P. M., Niederhuber J. E. Bone marrow-derived macrophage as accessory cells in antigen-induced T cell proliferation. H-2I region requirements for L-glutamic60-L-alanine30-L-tyrosine10 response. J Immunol. 1983 Feb;130(2):661–664. [PubMed] [Google Scholar]
- Snyderman R., Pike M. C. An inhibitor of macrophage chemotaxis produced by neoplasms. Science. 1976 Apr 23;192(4237):370–372. doi: 10.1126/science.946556. [DOI] [PubMed] [Google Scholar]
- Stadecker M. J., Ebner S. A. Characterization of mononuclear phagocytes in schistosomal egg granulomas of athymic mice. J Immunol. 1984 Oct;133(4):2231–2236. [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982 Dec 1;156(6):1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Oppenheim J. J. Regulation of murine macrophage Ia-antigen expression by products of activated spleen cells. J Exp Med. 1980 Dec 1;152(6):1734–1744. doi: 10.1084/jem.152.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. C., Beetham K. L. Cytocidal activity and proliferative ability of macrophages infiltrating the EMT6 tumor. Int J Cancer. 1978 Aug 15;22(2):152–159. doi: 10.1002/ijc.2910220208. [DOI] [PubMed] [Google Scholar]
- Stewart C. C. Local proliferation of mononuclear phagocytes in tumors. J Reticuloendothel Soc. 1983 Jul;34(1):23–27. [PubMed] [Google Scholar]
- Unanue E. R., Beller D. I., Lu C. Y., Allen P. M. Antigen presentation: comments on its regulation and mechanism. J Immunol. 1984 Jan;132(1):1–5. [PubMed] [Google Scholar]
- Wewers M. D., Rennard S. I., Hance A. J., Bitterman P. B., Crystal R. G. Normal human alveolar macrophages obtained by bronchoalveolar lavage have a limited capacity to release interleukin-1. J Clin Invest. 1984 Dec;74(6):2208–2218. doi: 10.1172/JCI111647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisler R. L., Newhouse Y. G., Lachman L. B. Heterogeneity of human monocyte subsets in the promotion of B cell colonies and the role of interleukin 1. J Immunol. 1982 Aug;129(2):455–460. [PubMed] [Google Scholar]


