Abstract
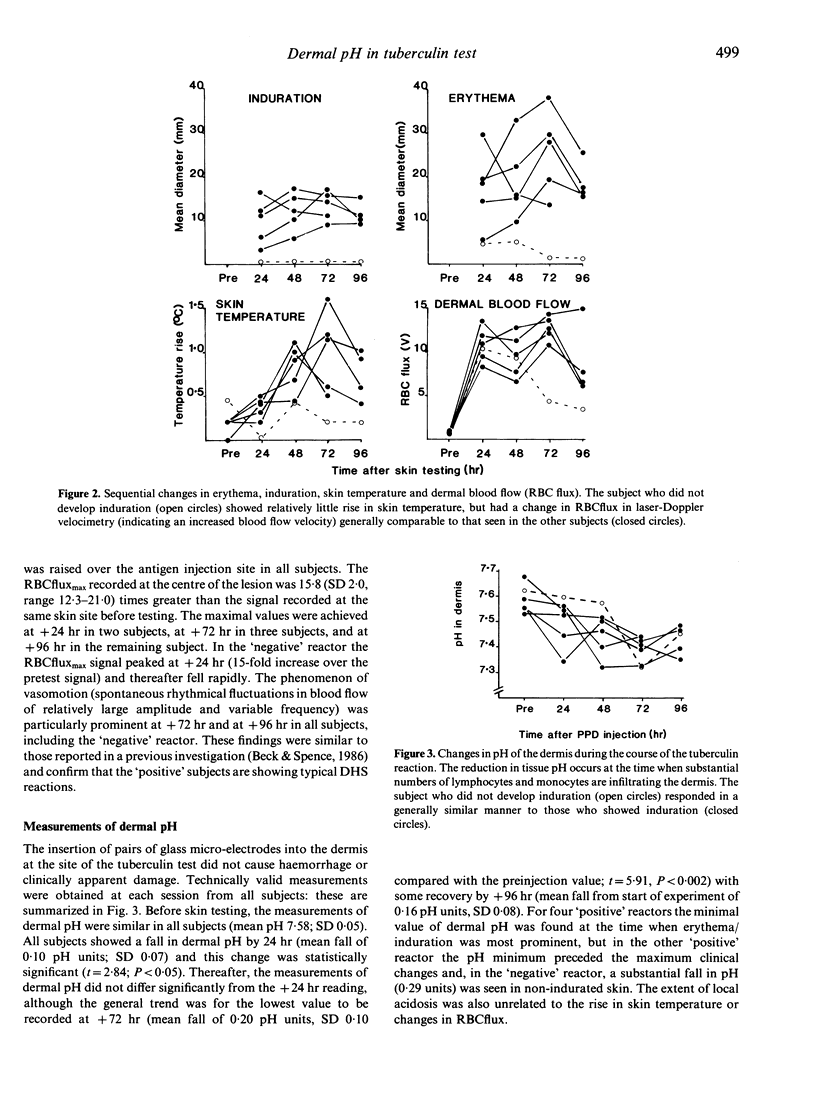
The response of six healthy young adults to tuberculin skin testing was studied. Five subjects developed a typical delayed-type hypersensitivity reaction to PPD with a local rise in skin temperature, and the sixth showed a less intense response; a considerable increase in blood flow velocity was seen in all reactions. All subjects showed a fall in pH in the dermis during the course of the reaction: in four subjects the pH minimum occurred at the time when the changes of erythema and induration were most prominent, in one subject the pH fall preceded the maximal clinical changes, and in the remaining subject a substantial fall in pH occurred with only transient erythema. It was concluded that the local tissue acidosis had resulted from the greatly increased metabolic demand of the lymphocytes and monocytes attracted into the dermis as part of the type IV delayed-type hypersensitivity reaction, and that the concurrent reactive hyperaemia was insufficient to clear the acidic metabolic products of the greatly increased cell population.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J. S., Spence V. A., Lowe J. G., Gibbs J. H. Measurement of skin swelling in the tuberculin test by ultrasonography. J Immunol Methods. 1986 Jan 22;86(1):125–130. doi: 10.1016/0022-1759(86)90275-9. [DOI] [PubMed] [Google Scholar]
- Coghill G., Gibbs J. H., Lowe J. G., Swanson Beck J. Cryopreservation with glycerol during cryostat sectioning for localisation of lymphocytes and accessory cell phenotypic subsets in tissue biopsies. J Clin Pathol. 1985 Jul;38(7):840–842. doi: 10.1136/jcp.38.7.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. H., Ferguson J., Brown R. A., Kenicer K. J., Potts R. C., Coghill G., Swanson Beck J. Histometric study of the localisation of lymphocyte subsets and accessory cells in human Mantoux reactions. J Clin Pathol. 1984 Nov;37(11):1227–1234. doi: 10.1136/jcp.37.11.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. K., Walker W. F. Micro-electrode measurement of skin pH in humans during ischaemia, hypoxia and local hypothermia. J Physiol. 1979 Jun;291:339–350. doi: 10.1113/jphysiol.1979.sp012817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. K., Walker W. F. Tissue pH electrodes for clinical applications. J Med Eng Technol. 1980 Jan;4(1):3–7. doi: 10.3109/03091908009161079. [DOI] [PubMed] [Google Scholar]
- Mihm S., Dröge W. Regulation of cytotoxic T-lymphocyte activation by L-lactate and pyruvate. Cell Immunol. 1985 Nov;96(1):235–240. doi: 10.1016/0008-8749(85)90355-7. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Grant B. W., Eddy A. A., Michael A. F. Immune cell populations in cutaneous delayed-type hypersensitivity. J Exp Med. 1983 Oct 1;158(4):1227–1242. doi: 10.1084/jem.158.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Seymour G. J., Duke O., Janossy G., Panayi G. Immunohistological analysis of delayed-type hypersensitivity in man. Cell Immunol. 1982 Dec;74(2):358–369. doi: 10.1016/0008-8749(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Scheynius A., Klareskog L., Forsum U. In situ identification of T lymphocyte subsets and HLA-DR expressing cells in the human skin tuberculin reaction. Clin Exp Immunol. 1982 Aug;49(2):325–330. [PMC free article] [PubMed] [Google Scholar]
- Sokal J. E. Editorial: Measurement of delayed skin-test responses. N Engl J Med. 1975 Sep 4;293(10):501–502. doi: 10.1056/NEJM197509042931013. [DOI] [PubMed] [Google Scholar]
- Swanson Beck J., Spence V. A. Patterns of blood flow in the microcirculation of the skin during the course of the tuberculin reaction in normal human subjects. Immunology. 1986 Jun;58(2):209–215. [PMC free article] [PubMed] [Google Scholar]
- Wang T., Foker J. E., Tsai M. Y. The shift of an increase in phosphofructokinase activity from protein synthesis-dependent to -independent mode during concanavalin A induced lymphocyte proliferation. Biochem Biophys Res Commun. 1980 Jul 16;95(1):13–19. doi: 10.1016/0006-291x(80)90697-x. [DOI] [PubMed] [Google Scholar]
- Wang T., Marquardt C., Foker J. Aerobic glycolysis during lymphocyte proliferation. Nature. 1976 Jun 24;261(5562):702–705. doi: 10.1038/261702a0. [DOI] [PubMed] [Google Scholar]



