Abstract
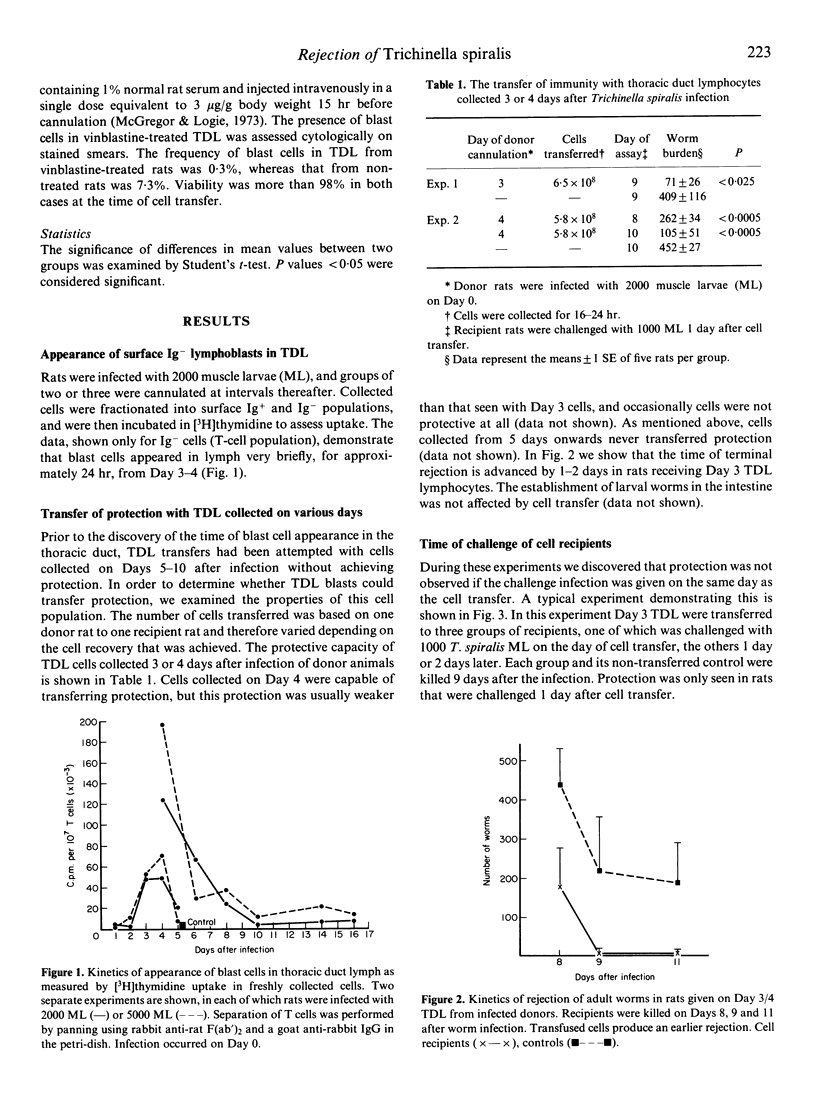
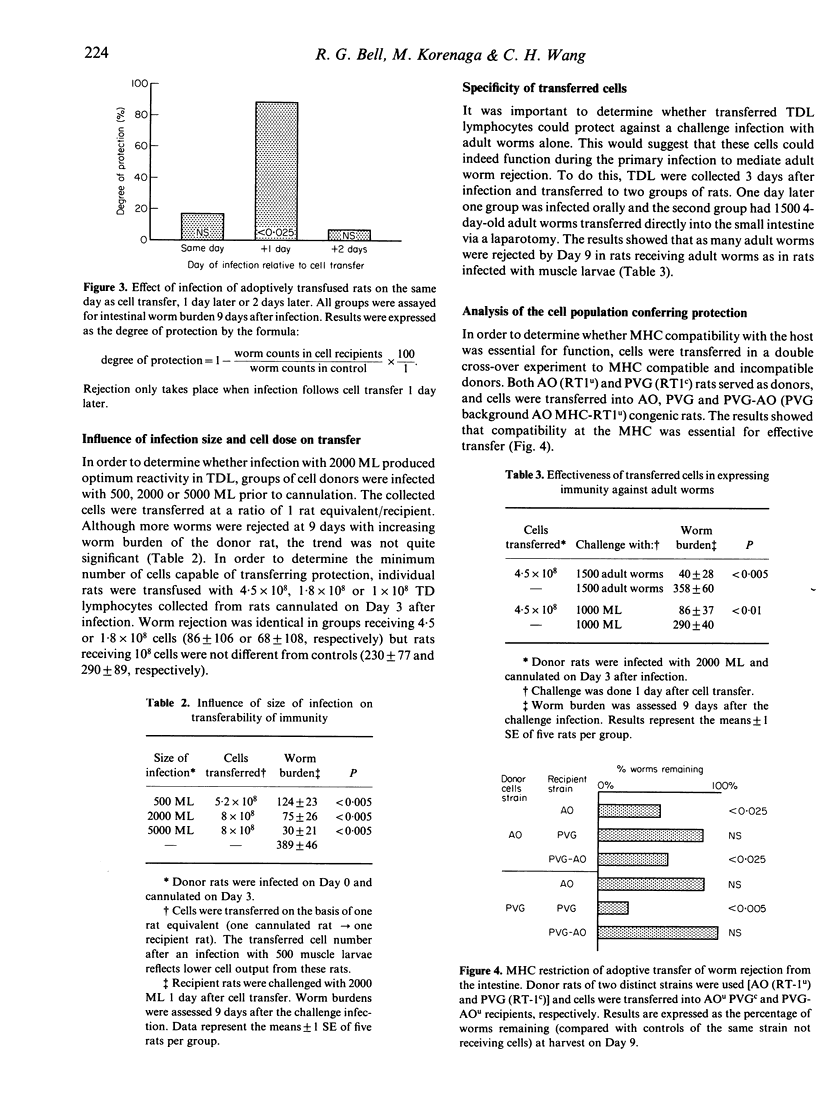
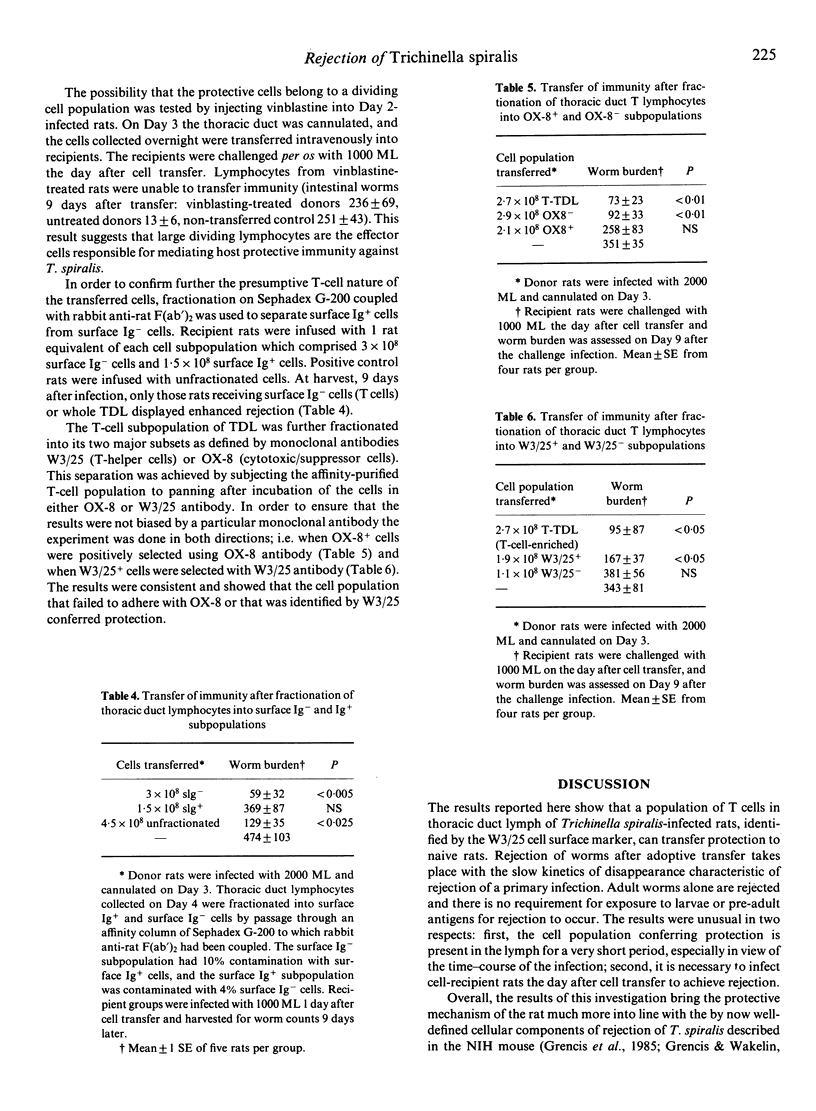
In Trichinella spiralis-infected rats, a population of cells in thoracic duct lymph (TDL) that can adoptively transfer protection to naive rats was identified and characterized. During the course of T. spiralis infection, blast cells appeared in lymph from Day 3-4, and only Day 3-4 TDL cells had protective properties after transfer. Protection was evident in a 1-2-day increase in the slow rejection of adult worms beginning 8-9 days after the challenge infection. The minimum number of TDL cells capable of transferring protection was 1.8 X 10(8) cells. Transferred cells could protect against a challenge infection with adult worms alone. A double cross-over experiment demonstrated that major histocompatibility complex identity was essential for effective transfer of protection (MHC restriction). An experiment using the mitotic inhibitor vinblastine showed that the protective cells belonged to a dividing cell population. The phenotype of the protective TDL was confirmed by a two-step cell separation procedure. First, it was demonstrated that surface Ig- cells (T cells) separated by affinity chromatography could transfer protection. Second, these surface Ig- cells were divided into two subpopulations by panning using monoclonal antibodies OX-8 and W3/25. The results showed that W3/25+ or OX-8- cells (T-helper) were effective in transferring protection. Protection was only seen when rats adoptively transferred with cells were challenged 1 day after cell transfer.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N. The localization of populations of lymphocytes defined by monoclonal antibodies in rat lymphoid tissues. Immunology. 1981 Apr;42(4):593–600. [PMC free article] [PubMed] [Google Scholar]
- Bell R. G., McGregor D. D., Adams L. S. Trichinella spiralis: characterization and strain distribution of rapid expulsion in inbred mice. Exp Parasitol. 1982 Jun;53(3):301–314. doi: 10.1016/0014-4894(82)90073-x. [DOI] [PubMed] [Google Scholar]
- Bell R. G., McGregor D. D., Despommier D. D. Trichinella spiralis: mediation of the intestinal component of protective immunity in the rat by multiple, phase-specific, antiparasitic responses. Exp Parasitol. 1979 Apr;47(2):140–157. doi: 10.1016/0014-4894(79)90068-7. [DOI] [PubMed] [Google Scholar]
- Bell R. G., McGregor D. D. Requirement for two discrete stimuli for induction of the intestinal rapid expulsion response against Trichinella spiralis in rats. Infect Immun. 1980 Jul;29(1):186–193. doi: 10.1128/iai.29.1.186-193.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- CHIPMAN P. B. The antigenic role of the excretions and secretions of adult Trichinella spiralis in the production of immunity in mice. J Parasitol. 1957 Dec;43(6):593–598. [PubMed] [Google Scholar]
- Crum E. D., Despommier D. D., McGregor D. D. Immunity to Trichinella spiralis. I. Transfer of resistance by two classes of lymphocytes. Immunology. 1977 Dec;33(6):787–795. [PMC free article] [PubMed] [Google Scholar]
- Crum E. D., McGregor D. D. Functional properties of T and B cells isolated by affinity chromatography from rat thoracic duct lymph. Cell Immunol. 1976 May;23(2):211–222. doi: 10.1016/0008-8749(76)90187-8. [DOI] [PubMed] [Google Scholar]
- Despommier D. D., McGregor D. D., Crum E. D., Carter P. B. Immunity to Trichinella spiralis. II. Expression of immunity against adult worms. Immunology. 1977 Dec;33(6):797–805. [PMC free article] [PubMed] [Google Scholar]
- Gamble H. R. Comparison of immune effects in mice immunized with Trichinella spiralis adult and larval antigens. J Parasitol. 1985 Oct;71(5):680–682. [PubMed] [Google Scholar]
- Grencis R. K., Riedlinger J., Wakelin D. L3T4-positive T lymphoblasts are responsible for transfer of immunity to Trichinella spiralis in mice. Immunology. 1985 Oct;56(2):213–218. [PMC free article] [PubMed] [Google Scholar]
- Grencis R. K., Wakelin D. Short lived, dividing cells mediate adoptive transfer of immunity to Trichinella spiralis in mice. I. Availability of cells in primary and secondary infections in relation to cellular changes in the mesenteric lymph node. Immunology. 1982 Jun;46(2):443–450. [PMC free article] [PubMed] [Google Scholar]
- LARSH J. E., Jr, GOULSON H. T., WEATHERLY N. F. STUDIES ON DELAYED (CELLULAR) HYPERSENSITIVITY IN MICE INFECTED WITH TRICHINELLA SPIRALIS. II. TRANSFER OF PERITONEAL EXUDATE CELLS. J Parasitol. 1964 Aug;50:496–498. [PubMed] [Google Scholar]
- McGregor D. D., Logie P. S. The mediator of cellular immunity. VI. Effect of the antimitotic drug vinblastine on the mediator of cellular resistance to infection. J Exp Med. 1973 Mar 1;137(3):660–674. doi: 10.1084/jem.137.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa Y., Miller H. R. Protection against Nippostrongylus brasiliensis by adoptive immunization with immune thoracic duct lymphocytes. Cell Immunol. 1978 Apr;37(1):51–60. doi: 10.1016/0008-8749(78)90173-9. [DOI] [PubMed] [Google Scholar]
- Ogilvie B. M., Love R. J., Jarra W., Brown K. N. Nippostrongylus brasiliensis infection in rats. The cellular requirement for worm expulsion. Immunology. 1977 Apr;32(4):521–528. [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M., Clark N. W. Stage specific secreted and somatic antigens of Trichinella spiralis. Mol Biochem Parasitol. 1983 Dec;9(4):319–327. doi: 10.1016/0166-6851(83)90088-9. [DOI] [PubMed] [Google Scholar]
- Silberstein D. S., Despommier D. D. Antigens from trichinella spiralis that induce a protective response in the mouse. J Immunol. 1984 Feb;132(2):898–904. [PubMed] [Google Scholar]
- Wakelin D., Wilson M. M. Immunity to Trichinella spiralis in irradiated mice. Int J Parasitol. 1980 Feb;10(1):37–41. doi: 10.1016/0020-7519(80)90062-4. [DOI] [PubMed] [Google Scholar]
- Wakelin D., Wilson M. M. T and B cells in the transfer of immunity against Trichinella spiralis in mice. Immunology. 1979 May;37(1):103–109. [PMC free article] [PubMed] [Google Scholar]
- Wakelin D., Wilson M. M. Transfer of immunity to Trichinella spiralis in the mouse with mesenteric lymph node cells: time of appearance of effective cells in donors and expression of immunity in recipients. Parasitology. 1977 Jun;74(3):215–224. doi: 10.1017/s0031182000047843. [DOI] [PubMed] [Google Scholar]


