Abstract
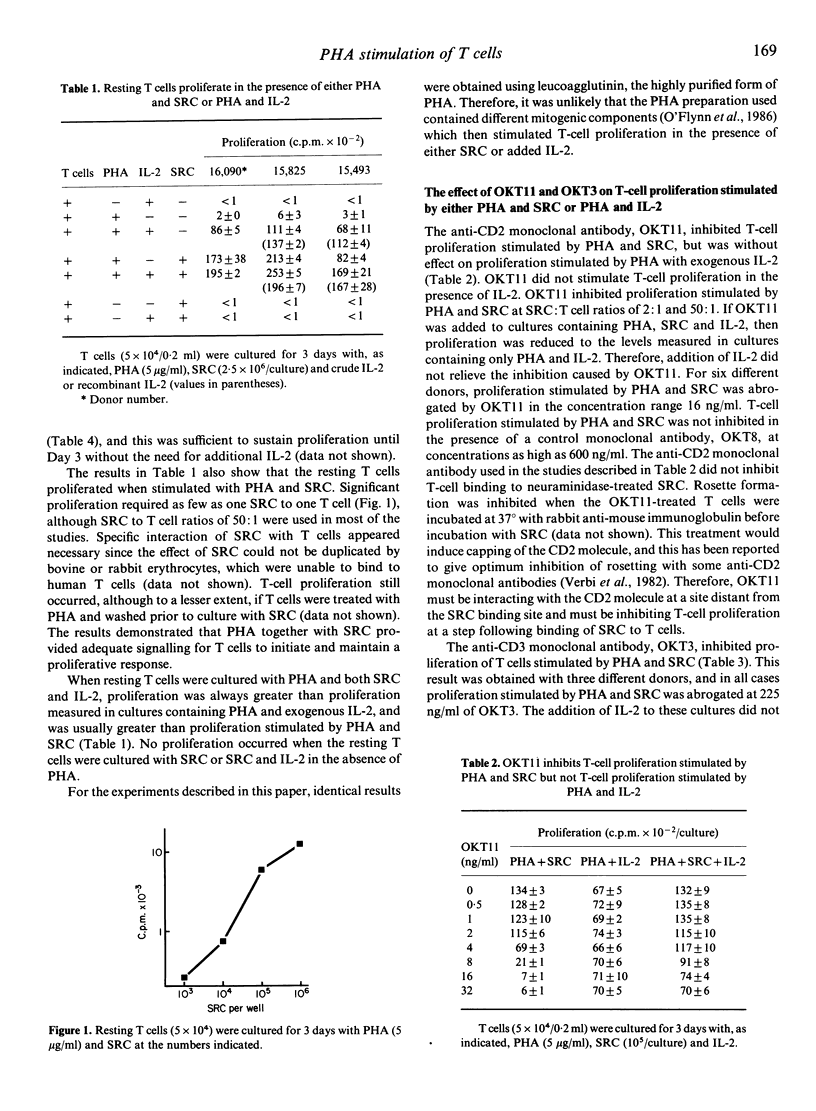
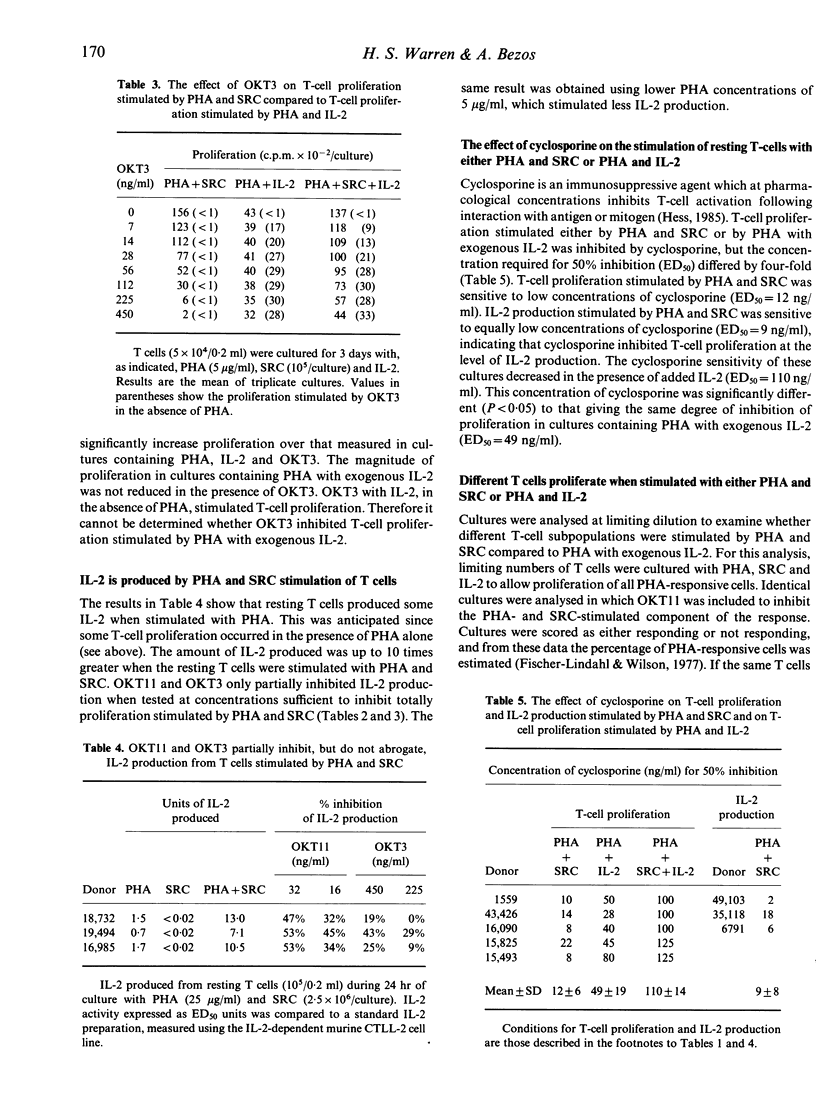
Data are presented showing that resting T cells proliferated in response to phytohaemagglutinin (PHA) provided that either sheep erythrocytes (SRC) or interleukin-2(IL-2) was added. Proliferation requiring PHA and SRC was inhibited by anti-CD2 monoclonal antibody (OKT11) and anti-CD3 monoclonal antibody (OKT3). These monoclonal antibodies only partially inhibited IL-2 production stimulated by PHA and SRC, and added IL-2 did not restore proliferation in these cultures. Low concentrations of cyclosporine inhibited both proliferation and IL-2 production (ED50 = 12 and 9 ng/ml, respectively) and this inhibition was partially relieved in the presence of added IL-2 (ED50 = 110 ng/ml). In contrast, proliferation stimulated by PHA with exogenous IL-2 was not inhibited in the presence of OKT11, and moderate concentrations of cyclosporine were required to inhibit proliferation (ED50 = 49 ng/ml). Evidence was obtained, from analysis of cultures at limiting dilution, to suggest that the different requirements for stimulation of PHA-responsive T cells reflected different T-cell subpopulations. The majority of PHA-responsive cells proliferated in the presence of PHA and SRC, whereas only a minority proliferated in the presence of PHA and exogenous IL-2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ebert E. C. Sheep red blood cells enhance T-lymphocyte proliferation. Clin Immunol Immunopathol. 1985 Nov;37(2):203–212. doi: 10.1016/0090-1229(85)90151-5. [DOI] [PubMed] [Google Scholar]
- Fox D. A., Hussey R. E., Fitzgerald K. A., Bensussan A., Daley J. F., Schlossman S. F., Reinherz E. L. Activation of human thymocytes via the 50KD T11 sheep erythrocyte binding protein induces the expression of interleukin 2 receptors on both T3+ and T3- populations. J Immunol. 1985 Jan;134(1):330–335. [PubMed] [Google Scholar]
- Fox D. A., Schlossman S. F., Reinherz E. L. Regulation of the alternative pathway of T cell activation by anti-T3 monoclonal antibody. J Immunol. 1986 Mar 15;136(6):1945–1950. [PubMed] [Google Scholar]
- Hünig T. R. The ligand of the erythrocyte receptor of T lymphocytes: expression on white blood cells and possible involvement in T cell activation. J Immunol. 1986 Mar 15;136(6):2103–2108. [PubMed] [Google Scholar]
- Hünig T. The cell surface molecule recognized by the erythrocyte receptor of T lymphocytes. Identification and partial characterization using a monoclonal antibody. J Exp Med. 1985 Sep 1;162(3):890–901. doi: 10.1084/jem.162.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzen D., Chu E., Terhost C., Leung D. Y., Gesner M., Miller R. A., Geha R. S. Mechanisms of human T cell response to mitogens: IL 2 induces IL 2 receptor expression and proliferation but not IL 2 synthesis in PHA-stimulated T cells. J Immunol. 1985 Sep;135(3):1840–1845. [PubMed] [Google Scholar]
- Kruger G., Welte K., Ciobanu N., Cunningham-Rundles C., Ralph P., Venuta S., Feldman S., Koziner B., Wang C. Y., Moore M. A. Interleukin-2 correction of defective in vitro T-cell mitogenesis in patients with common varied immunodeficiency. J Clin Immunol. 1984 Jul;4(4):295–303. doi: 10.1007/BF00915297. [DOI] [PubMed] [Google Scholar]
- Lindahl K. F., Wilson D. B. Histocompatibility antigen-activated cytotoxic T lymphocytes. II. Estimates of the frequency and specificity of precursors. J Exp Med. 1977 Mar 1;145(3):508–522. doi: 10.1084/jem.145.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- O'Flynn K., Krensky A. M., Beverley P. C., Burakoff S. J., Linch D. C. Phytohaemagglutinin activation of T cells through the sheep red blood cell receptor. Nature. 1985 Feb 21;313(6004):686–687. doi: 10.1038/313686a0. [DOI] [PubMed] [Google Scholar]
- Palacios R., Martinez-Maza O. Is the E receptor on human T lymphocytes a "negative signal receptor"? J Immunol. 1982 Dec;129(6):2479–2485. [PubMed] [Google Scholar]
- Reed J. C., Tadmori W., Kamoun M., Koretzky G., Nowell P. C. Suppression of interleukin 2 receptor acquisition by monoclonal antibodies recognizing the 50 KD protein associated with the sheep erythrocyte receptor on human T lymphocytes. J Immunol. 1985 Mar;134(3):1631–1639. [PubMed] [Google Scholar]
- Tadmori W., Kant J. A., Kamoun M. Down regulation of IL 2 mRNA by antibody to the 50-kd protein associated with E receptors on human T lymphocyte. J Immunol. 1986 Feb 15;136(4):1155–1160. [PubMed] [Google Scholar]
- Tadmori W., Reed J. C., Nowell P. C., Kamoun M. Functional properties of the 50 kd protein associated with the E-receptor on human T lymphocytes: suppression of IL 2 production by anti-p50 monoclonal antibodies. J Immunol. 1985 Mar;134(3):1709–1716. [PubMed] [Google Scholar]
- Verbi W., Greaves M. F., Schneider C., Koubek K., Janossy G., Stein H., Kung P., Goldstein G. Monoclonal antibodies OKT 11 and OKT 11A have pan-T reactivity and block sheep erythrocyte "receptors". Eur J Immunol. 1982 Jan;12(1):81–86. doi: 10.1002/eji.1830120115. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Atkinson K., Pembrey R. G., Biggs J. C. Human bone marrow allograft recipients: production of, and responsiveness to, interleukin 2. J Immunol. 1983 Oct;131(4):1771–1775. [PubMed] [Google Scholar]
- Warren H. S. Differentiation of NK-like cells from OKT3-, OKT11+, and OKM1+ small resting lymphocytes by culture with autologous T cell blasts and lymphokine. J Immunol. 1984 Jun;132(6):2888–2894. [PubMed] [Google Scholar]
- Warren H. S., Pembrey R. G. A method for the production and quantitative assay of human lymphokine preparations. J Immunol Methods. 1981;41(1):9–21. doi: 10.1016/0022-1759(81)90269-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson M., Morris A. G. Role of the E receptor in interferon-gamma expression: sheep erythrocytes augment interferon-gamma production by human lymphocytes. Cell Immunol. 1984 Jun;86(1):109–117. doi: 10.1016/0008-8749(84)90364-2. [DOI] [PubMed] [Google Scholar]


