Abstract
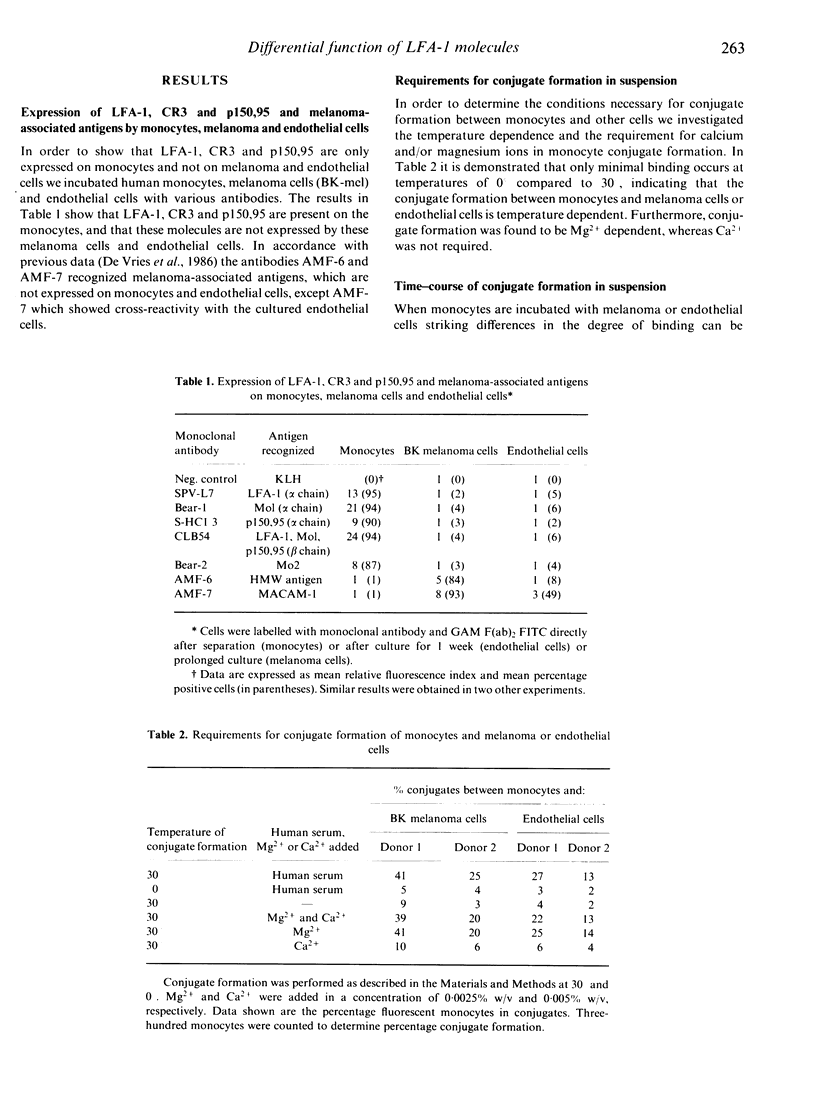
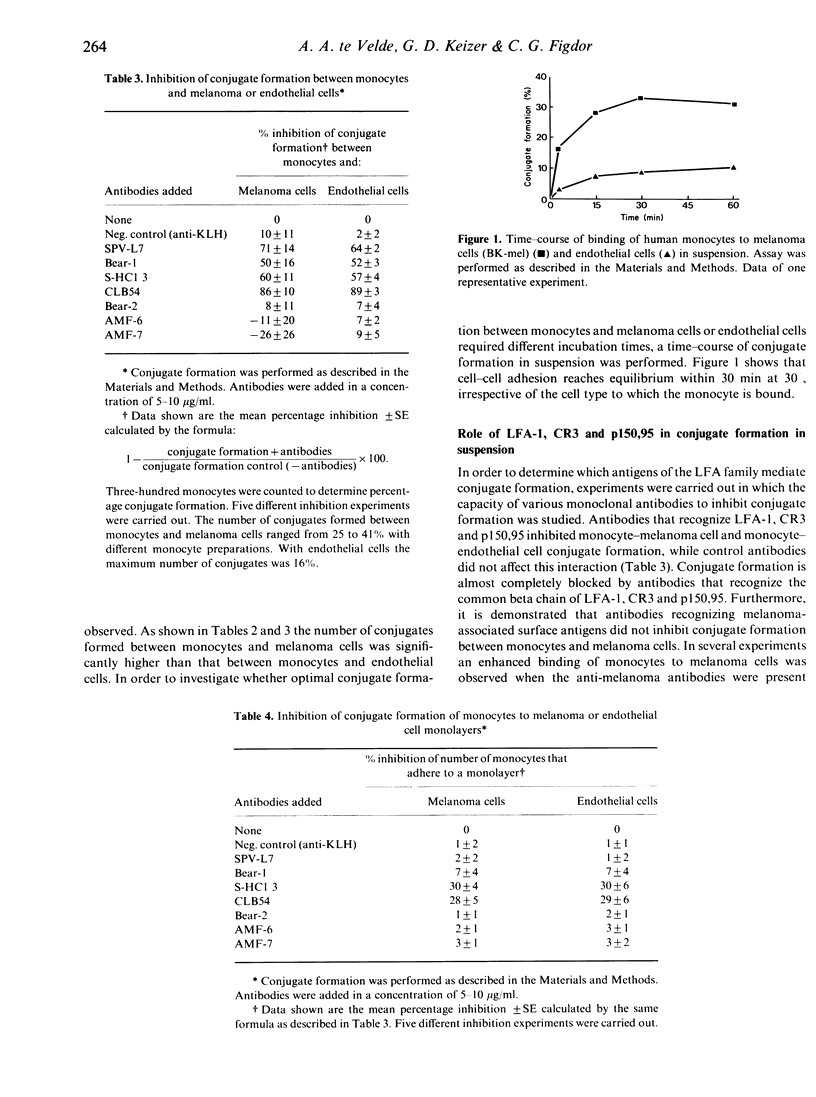
Human peripheral blood monocytes from normal, healthy donors express the leucocyte function-associated antigen (LFA)-1, CR3 and p150,95. These heterodimeric antigens are members of a glycoprotein family sharing a common beta subunit but endowed with distinct alpha chains. They have been shown to play an important role in cell-cell interactions. In the present study we have investigated the role of these molecules in the interaction of monocytes with endothelial cells and melanoma (tumour) cells. Heterotypic cell-cell interactions were studied in single cell conjugate assays and by adhesion of monocytes to monolayers of cells. The results demonstrate that monoclonal antibodies directed against LFA-1 alpha, CR3 alpha, p150,95 alpha and the common beta chain strongly reduce the number of conjugates (71, 50, 60 and 89% inhibition, respectively), formed between monocytes and melanoma or endothelial cells in a single cell assay. In contrast, adhesion of monocytes to monolayers of the same cells seems only to depend on p150,95, since only antibodies directed to the alpha chain of this molecule and to the common beta chain inhibited adhesion. Interestingly, the number of conjugates formed with melanoma cells in single cell assays was at least twice the number of conjugates formed between monocytes and endothelial cells, whereas no differences were observed in the adhesion of monocytes to monolayers of these cells. However, the basis for this phenomenon is not yet clear. These results indicate that not only LFA-1 but also CR3 and p150,95 can mediate adhesion to target cells in suspension, but that monocyte adhesion to monolayers is caused by a different mechanism in which the p150,95 molecule seems to play a prominent role.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Miller L. J., Schmalstieg F. C., Rothlein R., Springer T. A. Contributions of the Mac-1 glycoprotein family to adherence-dependent granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. J Immunol. 1986 Jul 1;137(1):15–27. [PubMed] [Google Scholar]
- Arnaout M. A., Todd R. F., 3rd, Dana N., Melamed J., Schlossman S. F., Colten H. R. Inhibition of phagocytosis of complement C3- or immunoglobulin G-coated particles and of C3bi binding by monoclonal antibodies to a monocyte-granulocyte membrane glycoprotein (Mol). J Clin Invest. 1983 Jul;72(1):171–179. doi: 10.1172/JCI110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavida B., Bradley T. P., Grimm E. A. Frequency determination of killer cells by a single-cell cytotoxic assay. Methods Enzymol. 1983;93:270–280. doi: 10.1016/s0076-6879(83)93049-5. [DOI] [PubMed] [Google Scholar]
- Chen W. T., Singer S. J. Immunoelectron microscopic studies of the sites of cell-substratum and cell-cell contacts in cultured fibroblasts. J Cell Biol. 1982 Oct;95(1):205–222. doi: 10.1083/jcb.95.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figdor C. G., Van Es W. L., Leemans J. M., Bont W. S. A centrifugal elutriation system of separating small numbers of cells. J Immunol Methods. 1984 Mar 30;68(1-2):73–87. doi: 10.1016/0022-1759(84)90138-8. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Senecal F. M., Schwartz B. R., Yee E. K., Taylor R. F., Beatty P. G., Price T. H., Ochs H. D. The role of neutrophil membrane glycoprotein GP-150 in neutrophil adherence to endothelium in vitro. Blood. 1985 Jul;66(1):167–178. [PubMed] [Google Scholar]
- Keizer G. D., Borst J., Figdor C. G., Spits H., Miedema F., Terhorst C., De Vries J. E. Biochemical and functional characteristics of the human leukocyte membrane antigen family LFA-1, Mo-1 and p150,95. Eur J Immunol. 1985 Nov;15(11):1142–1148. doi: 10.1002/eji.1830151114. [DOI] [PubMed] [Google Scholar]
- Keizer G. D., Figdor C. G., De Vries J. E. Sensitive and quantitative determination of monocyte adherence. J Immunol Methods. 1986 Dec 4;95(1):141–147. doi: 10.1016/0022-1759(86)90329-7. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Herberman R. B. The current status of human monocyte-mediated cytotoxicity against tumor cells. J Leukoc Biol. 1985 Sep;38(3):441–445. doi: 10.1002/jlb.38.3.441. [DOI] [PubMed] [Google Scholar]
- Krensky A. M., Robbins E., Springer T. A., Burakoff S. J. LFA-1, LFA-2, and LFA-3 antigens are involved in CTL-target conjugation. J Immunol. 1984 May;132(5):2180–2182. [PubMed] [Google Scholar]
- Lanier L. L., Arnaout M. A., Schwarting R., Warner N. L., Ross G. D. p150/95, Third member of the LFA-1/CR3 polypeptide family identified by anti-Leu M5 monoclonal antibody. Eur J Immunol. 1985 Jul;15(7):713–718. doi: 10.1002/eji.1830150714. [DOI] [PubMed] [Google Scholar]
- Miedema F., Tetteroo P. A., Hesselink W. G., Werner G., Spits H., Melief C. J. Both Fc receptors and lymphocyte-function-associated antigen 1 on human T gamma lymphocytes are required for antibody-dependent cellular cytotoxicity (killer cell activity). Eur J Immunol. 1984 Jun;14(6):518–523. doi: 10.1002/eji.1830140607. [DOI] [PubMed] [Google Scholar]
- Pawlowski N. A., Abraham E. L., Pontier S., Scott W. A., Cohn Z. A. Human monocyte-endothelial cell interaction in vitro. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8208–8212. doi: 10.1073/pnas.82.23.8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. E., Bartley G., Levine H., Schlossman S. F., Ritz J. Functional characterization of LFA-1 antigens in the interaction of human NK clones and target cells. J Immunol. 1985 Aug;135(2):1020–1025. [PubMed] [Google Scholar]
- Schwarting R., Stein H., Wang C. Y. The monoclonal antibodies alpha S-HCL 1 (alpha Leu-14) and alpha S-HCL 3 (alpha Leu-M5) allow the diagnosis of hairy cell leukemia. Blood. 1985 Apr;65(4):974–983. [PubMed] [Google Scholar]
- Spits H., van Schooten W., Keizer H., van Seventer G., van de Rijn M., Terhorst C., de Vries J. E. Alloantigen recognition is preceded by nonspecific adhesion of cytotoxic T cells and target cells. Science. 1986 Apr 18;232(4748):403–405. doi: 10.1126/science.3485822. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Thompson W. S., Miller L. J., Schmalstieg F. C., Anderson D. C. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J Exp Med. 1984 Dec 1;160(6):1901–1918. doi: 10.1084/jem.160.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassmann G., Springer T. A., Somers S. D., Adams D. O. Mechanisms of tumor cell capture by activated macrophages: evidence for involvement of lymphocyte function-associated (LFA)-1 antigen. J Immunol. 1986 Jun 1;136(11):4328–4333. [PubMed] [Google Scholar]
- Tax W. J., Hermes F. F., Willems R. W., Capel P. J., Koene R. A. Fc receptors for mouse IgG1 on human monocytes: polymorphism and role in antibody-induced T cell proliferation. J Immunol. 1984 Sep;133(3):1185–1189. [PubMed] [Google Scholar]
- Wallis W. J., Beatty P. G., Ochs H. D., Harlan J. M. Human monocyte adherence to cultured vascular endothelium: monoclonal antibody-defined mechanisms. J Immunol. 1985 Oct;135(4):2323–2330. [PubMed] [Google Scholar]
- Yssel H., De Vries J. E., Koken M., Van Blitterswijk W., Spits H. Serum-free medium for generation and propagation of functional human cytotoxic and helper T cell clones. J Immunol Methods. 1984 Aug 3;72(1):219–227. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]
- de Vries J. E., Keizer G. D., te Velde A. A., Voordouw A., Ruiter D., Rümke P., Spits H., Figdor C. G. Characterization of melanoma-associated surface antigens involved in the adhesion and motility of human melanoma cells. Int J Cancer. 1986 Oct 15;38(4):465–473. doi: 10.1002/ijc.2910380403. [DOI] [PubMed] [Google Scholar]


