Abstract
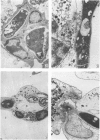
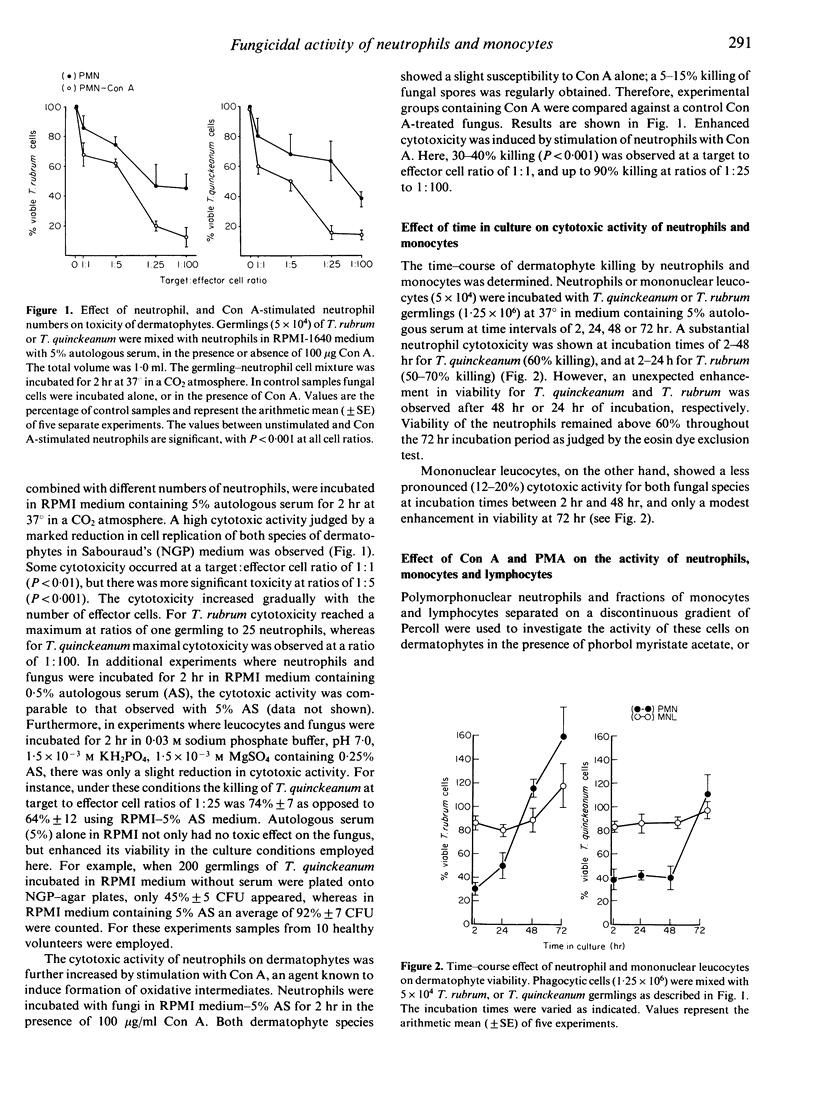
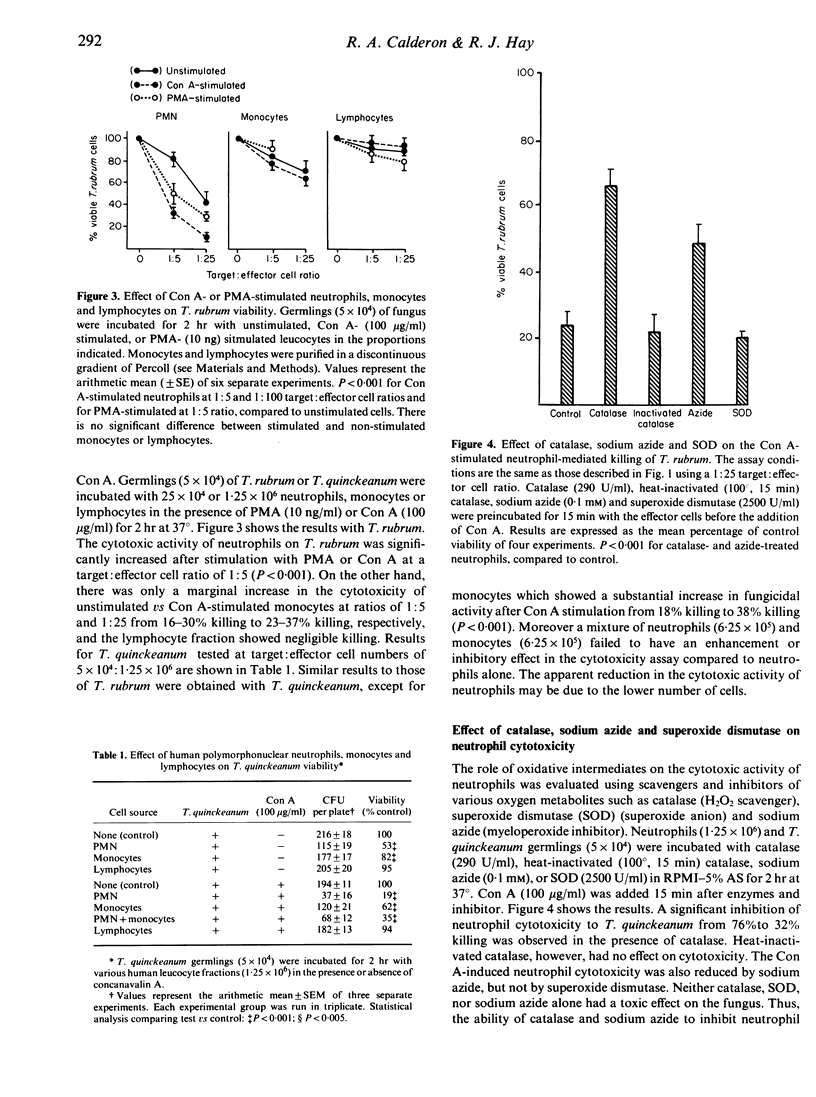
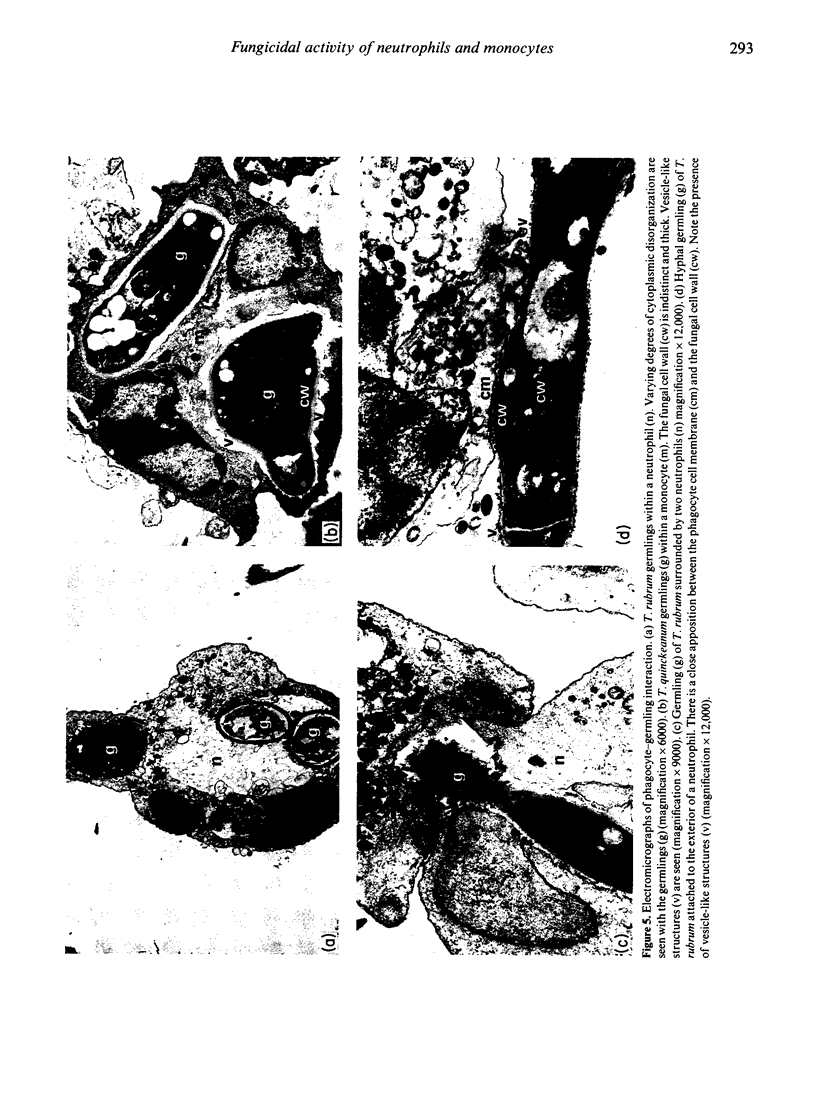
Human peripheral polymorphonuclear neutrophils exhibited potent cytotoxic activity against the dermatophyte fungi Tricophyton quinckeanum and T. rubrum as assessed by inhibition of fungal replication in Sabouraud's agar. Monocytes also showed cytotoxic activity, but this was less pronounced than that of neutrophils, while lymphocytes had no toxic effect. Cytotoxicity showed a linear relationship to the target cell:effector cell ratio, with significant killing detected at a ratio of one neutrophil to one fungal cell. Fungal killing was optimal at incubation times of 2-24 hr for T. rubrum and 2-48 hr for T. quinckeanum. Thereafter, neutrophils were unable to prevent fungal replication while remaining viable. cytotoxicity was markedly reduced by sodium azide, an agent that inhibits haem enzymes, and by catalase, but not by heat-inactivated catalase or superoxide dismutase. The fungicidal activity of neutrophils and monocytes was greatly increased by stimulation with phorbol myristate acetate (PMA) or with concanavalin A (Con A) compounds known to stimulate the secretion of lysosomal enzymes and the production of highly reactive oxygen intermediates. The cytotoxic activity of monocytes to T. quinckeanum, but not to T. rubrum, was also increased by Con A treatment. Neutrophil and monocyte phagocytosis of dermatophytes was demonstrated by electron microscopy studies. Disrupted T. quinckeanum and T. rubrum germlings were identified in the cytoplasm of the phagocytic cells, and similarly disruption of hyphae surrounded, but not engulfed, by neutrophils was also observed. These studies suggest that phagocytosis and/or oxidative products of the respiratory burst of neutrophils and monocytes may be implicated in the killing of dermatophytes in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Sumidaie A. M., Jones D. L., Young H. L. Characterisation of the under-agarose method for quantifying migration of highly purified human monocytes. J Immunol Methods. 1984 Dec 14;75(1):129–140. doi: 10.1016/0022-1759(84)90232-1. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Calderon R. A., Hay R. J. Cell-mediated immunity in experimental murine dermatophytosis. II. Adoptive transfer of immunity to dermatophyte infection by lymphoid cells from donors with acute or chronic infections. Immunology. 1984 Nov;53(3):465–472. [PMC free article] [PubMed] [Google Scholar]
- Calderon R. A., Shennan G. I. Susceptibility of Trichophyton quinckeanum and Trichophyton rubrum to products of oxidative metabolism. Immunology. 1987 Jul;61(3):283–288. [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med. 1975 Jun 1;141(6):1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Isturiz R. E., Malech H. L., Root R. K., Wilfert C. M., Gutman L., Buckley R. H. Fungal infection in chronic granulomatous disease. The importance of the phagocyte in defense against fungi. Am J Med. 1981 Jul;71(1):59–66. doi: 10.1016/0002-9343(81)90259-x. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., Johnston R. B., Jr Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood. 1976 Apr;47(4):545–554. [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978 Feb;61(2):349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Hoffstein S. T., Weissmann G. Mechanisms of lysosomal enzyme release from human polymorphonuclear leukocytes. Effects of phorbol myristate acetate. J Cell Biol. 1975 Sep;66(3):647–652. doi: 10.1083/jcb.66.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. J., Calderon R. A., Collins M. J. Experimental dermatophytosis: the clinical and histopathologic features of a mouse model using Trichophyton quinckeanum (mouse favus). J Invest Dermatol. 1983 Sep;81(3):270–274. doi: 10.1111/1523-1747.ep12518292. [DOI] [PubMed] [Google Scholar]
- Hay R. J. Chronic dermatophyte infections. I. Clinical and mycological features. Br J Dermatol. 1982 Jan;106(1):1–7. doi: 10.1111/j.1365-2133.1982.tb00895.x. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975 Feb;55(2):338–346. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini B., Kaaman T. T-lymphocyte subpopulations in patients with chronic dermatophytosis. Int Arch Allergy Appl Immunol. 1981;66(1):105–109. doi: 10.1159/000232806. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Simchowitz L., Schur P. H. Lectin-dependent neutrophil-mediated cytotoxicity. I. Characteristics. Immunology. 1976 Aug;31(2):303–311. [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975 Aug 1;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]