Abstract
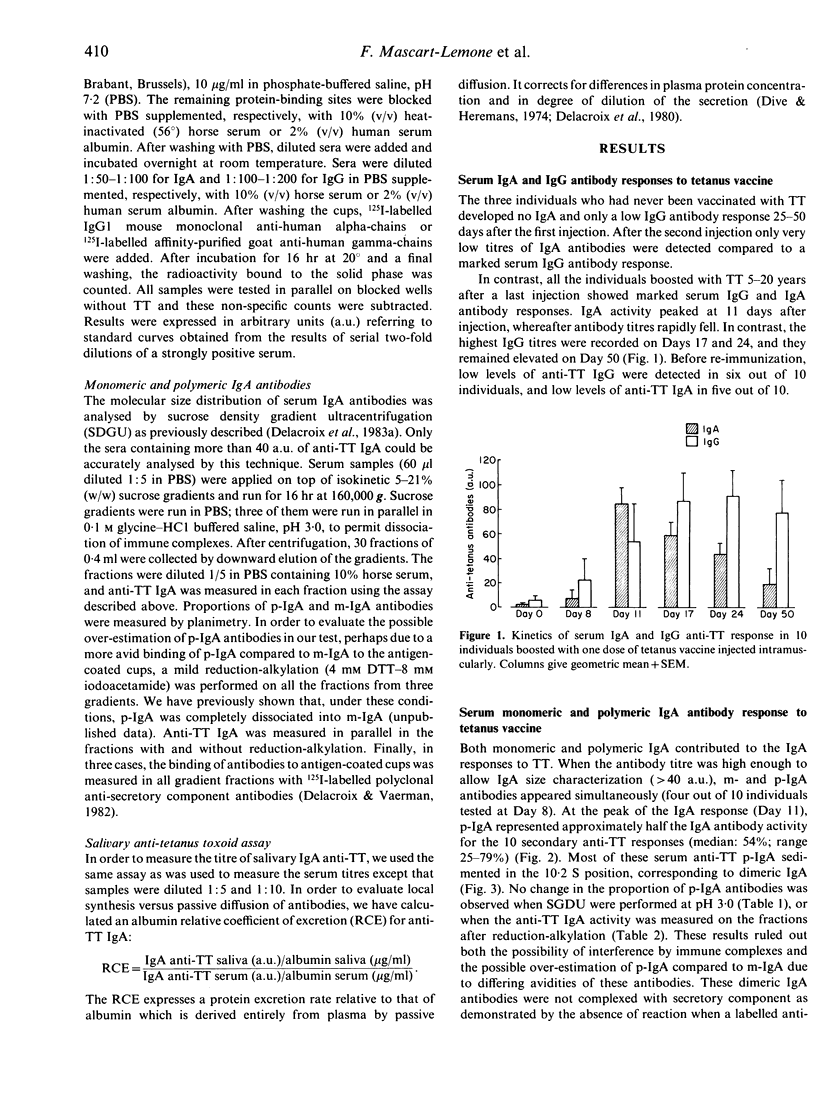
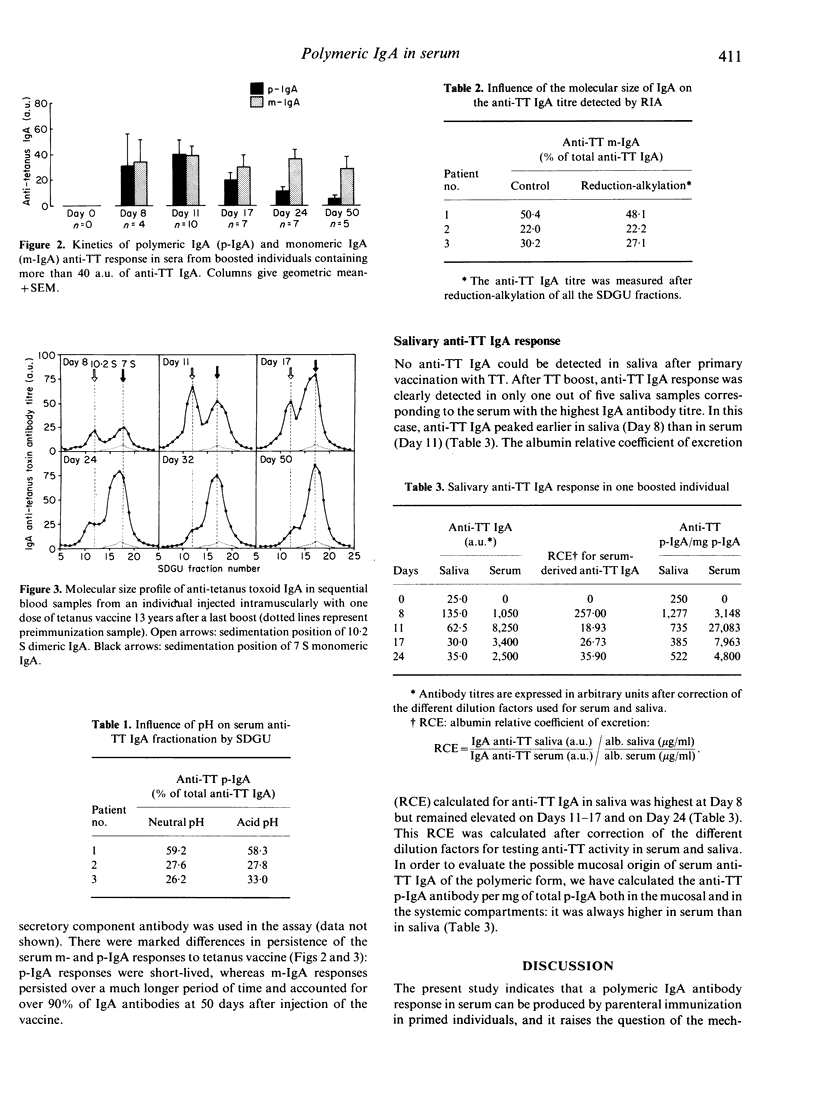
The magnitude and the kinetics of the serum-specific polymeric (p-) and monomeric (m-) IgA antibody responses were analysed following parenteral stimulation with tetanus toxoid (TT) vaccine in 10 volunteers, 5-20 years after a previous boost. A rapid marked serum IgA antibody response involving both the monomeric and polymeric components of IgA was observed: m-IgA and p-IgA antibodies reached a peak of serum activity at about 11 days, around 6 days before the peak of IgG antibody activity. At the peak of the IgA response, p-IgA accounted for approximately half of the anti-TT activity (median 54%, 25-79%). However, p-IgA antibodies rapidly disappeared from serum over a few weeks, whereas the serum m-IgA antibody response was maintained over a prolonged period of time. For one subject out of five, anti-TT IgA were also detected in saliva with a peak of activity earlier than in serum. Calculation of the albumin relative coefficient of excretion for anti-TT IgA in this saliva suggested a local synthesis of these antibodies. The present study indicates that a polymeric IgA antibody response in serum can be produced by parenteral immunization in primed individuals, and it raises the question of the mechanisms that control polymeric versus monomeric IgA production.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bene M. C., Faure G., Duheille J. IgA nephropathy: characterization of the polymeric nature of mesangial deposits by in vitro binding of free secretory component. Clin Exp Immunol. 1982 Mar;47(3):527–534. [PMC free article] [PubMed] [Google Scholar]
- Delacroix D. L., Elkom K. B., Geubel A. P., Hodgson H. F., Dive C., Vaerman J. P. Changes in size, subclass, and metabolic properties of serum immunoglobulin A in liver diseases and in other diseases with high serum immunoglobulin A. J Clin Invest. 1983 Feb;71(2):358–367. doi: 10.1172/JCI110777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacroix D. L., Hodgson H. J., McPherson A., Dive C., Vaerman J. P. Selective transport of polymeric immunoglobulin A in bile. Quantitative relationships of monomeric and polymeric immunoglobulin A, immunoglobulin M, and other proteins in serum, bile, and saliva. J Clin Invest. 1982 Aug;70(2):230–241. doi: 10.1172/JCI110610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacroix D. L., Liroux E., Vaerman J. P. High proportion of polymeric IgA in young infants' sera and independence between IgA-size and IgA-subclass distributions. J Clin Immunol. 1983 Jan;3(1):51–56. doi: 10.1007/BF00919138. [DOI] [PubMed] [Google Scholar]
- Delacroix D. L., Vaerman J. P. Secretory component (SC): preferential binding to heavy (greater than 11S) IgA polymers and IgM in serum, in contrast to predominance of 11S and free SC forms in secretions. Clin Exp Immunol. 1982 Sep;49(3):717–724. [PMC free article] [PubMed] [Google Scholar]
- Dive C., Heremans J. F. Nature and origin of the proteins of bile. I. A comparative analysis of serum and bile proteins in man. Eur J Clin Invest. 1974 Aug;4(4):235–239. doi: 10.1111/j.1365-2362.1974.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Kutteh W. H., Prince S. J., Mestecky J. Tissue origins of human polymeric and monomeric IgA. J Immunol. 1982 Feb;128(2):990–995. [PubMed] [Google Scholar]
- Mannhalter J. W., Neychev H. O., Zlabinger G. J., Ahmad R., Eibl M. M. Modulation of the human immune response by the non-toxic and non-pyrogenic adjuvant aluminium hydroxide: effect on antigen uptake and antigen presentation. Clin Exp Immunol. 1985 Jul;61(1):143–151. [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., Russell M. W., Jackson S., Brown T. A. The human IgA system: a reassessment. Clin Immunol Immunopathol. 1986 Jul;40(1):105–114. doi: 10.1016/0090-1229(86)90073-5. [DOI] [PubMed] [Google Scholar]
- Radl J., Schuit H. R., Mestecky J., Hijmans W. The origin of monomeric and polymeric forms of IgA in man. Adv Exp Med Biol. 1974;45(0):57–65. doi: 10.1007/978-1-4613-4550-3_7. [DOI] [PubMed] [Google Scholar]
- Skvaril F., Morell A. Distribution of IgA subclasses in sera and bone marrow plasma cells of 21 normal individuals. Adv Exp Med Biol. 1974;45(0):433–435. doi: 10.1007/978-1-4613-4550-3_51. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Gahnberg L., Taubman M. A., Ebersole J. L. Salivary antibody responses to oral and parenteral vaccines in children. J Clin Immunol. 1986 Jan;6(1):43–49. doi: 10.1007/BF00915363. [DOI] [PubMed] [Google Scholar]
- TOMASI T. B., Jr, TAN E. M., SOLOMON A., PRENDERGAST R. A. CHARACTERISTICS OF AN IMMUNE SYSTEM COMMON TO CERTAIN EXTERNAL SECRETIONS. J Exp Med. 1965 Jan 1;121:101–124. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth D. J., Payne A. W., Leonard J. N., Fry L., Holborow E. J. IgA in dermatitis-herpetiformis skin is dimeric. Lancet. 1982 Feb 27;1(8270):478–479. doi: 10.1016/s0140-6736(82)91452-0. [DOI] [PubMed] [Google Scholar]


