Abstract
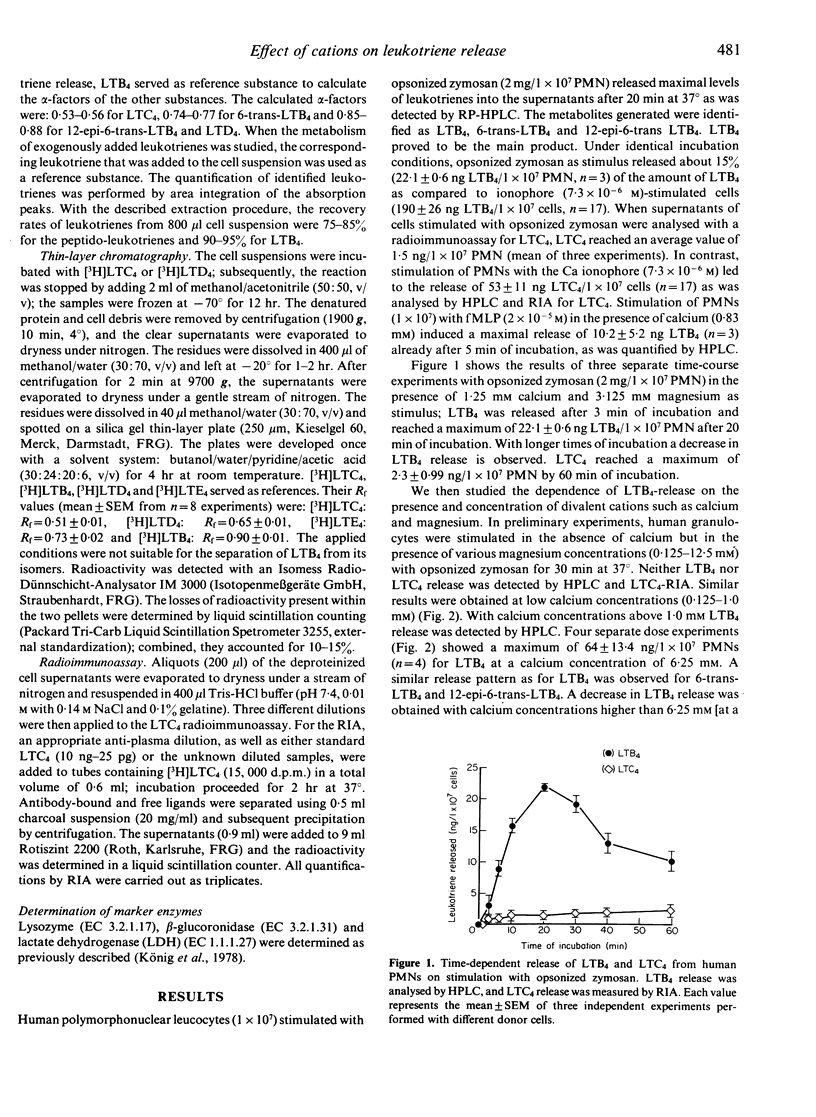
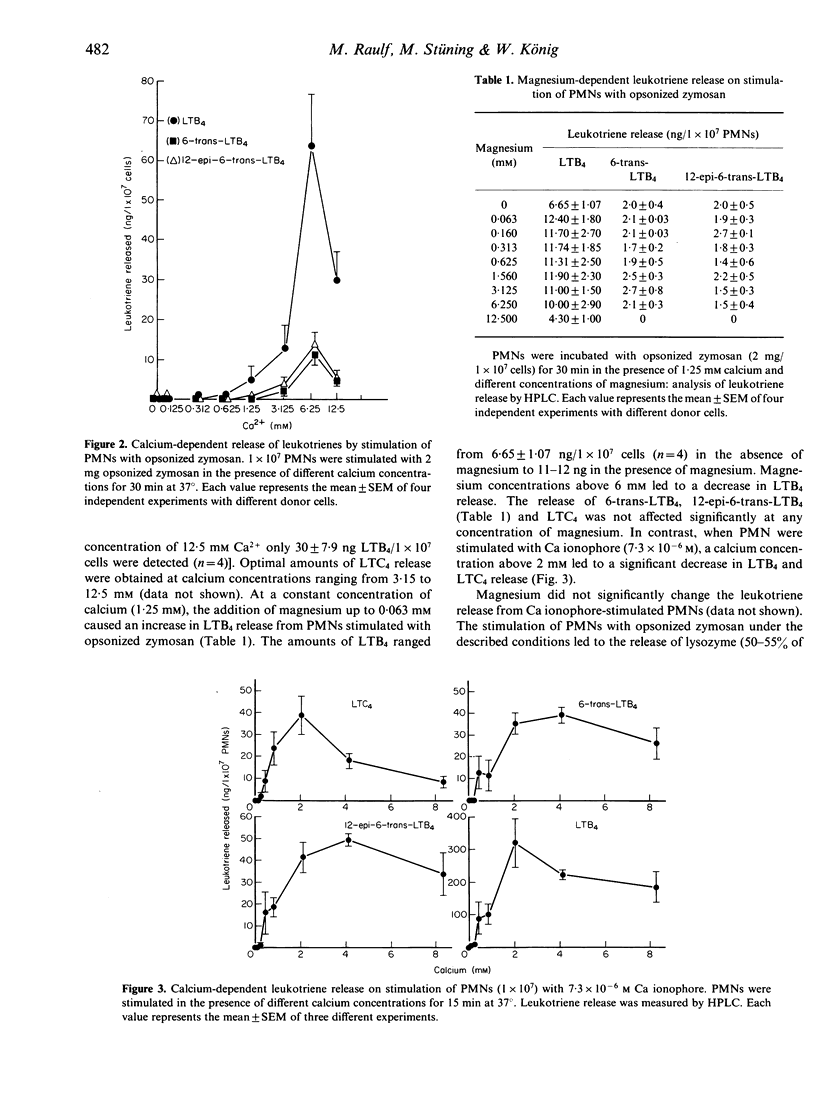
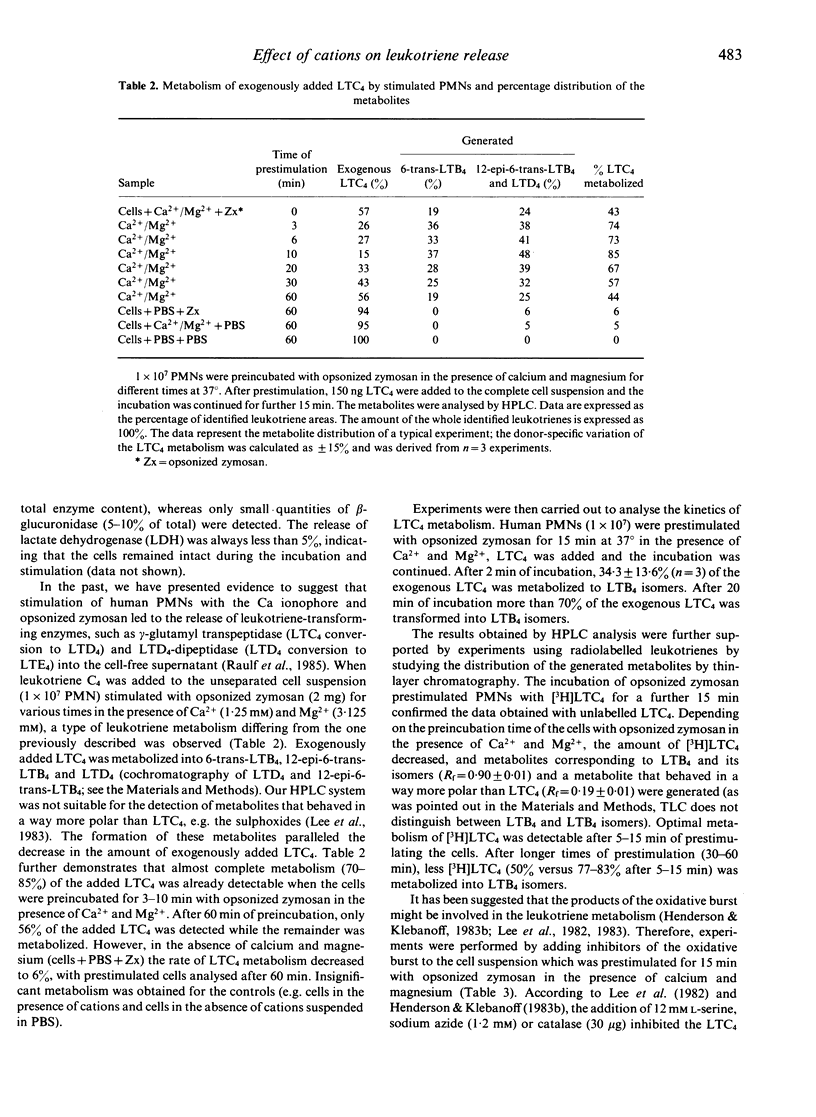
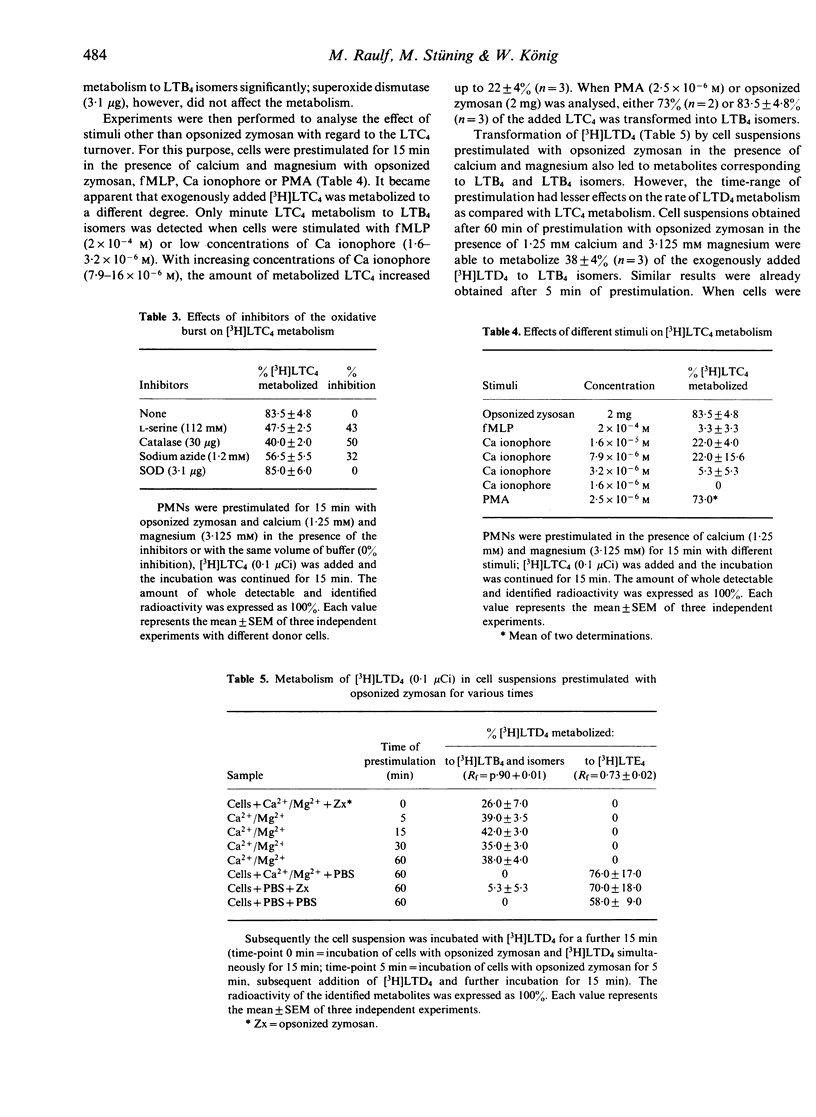
Stimulation of human polymorphonuclear granulocytes with opsonized zymosan leads to a time-dependent release of the leukotrienes LTB4, 6-trans-LTB4, 12-epi-6-trans-LTB4 and LTC4 measured by HPLC analysis and LTC4 radioimmunoassay. The amounts of leukotrienes released on stimulation with opsonized zymosan are lower than those obtained with the calcium ionophore A23187. Opsonized zymosan as stimulus required higher calcium concentrations to obtain optimal leukotriene release as compared with the calcium ionophore. Magnesium in the presence of calcium increased the leukotriene formation with opsonized zymosan. Addition of the peptido-leukotrienes LTC4, LTD4 to the unseparated, opsonized zymosan-prestimulated cell suspension leads to the generation of 6-trans-LTB4, 12-epi-6-trans-LTB4 and a metabolite which is more polar than LTC4. The rate of LTC4 metabolism is strongly dependent on the time of prestimulation as well as on the stimuli used for cell triggering, e.g. opsonized zymosan, phorbol-12-myristate-13-acetate, calcium ionophore A23187 or formyl-methionyl-leucyl-phenylalanine. Inhibitors of the oxidative burst decreased LTC4 metabolism. Thus, the peptido-leukotriene LTC4 is metabolized by two pathways: the enzymes gamma-glutamyl-transpeptidase and dipeptidase transform LTC4 into LTD4 and LTE4; these enzymes are present within the supernatants of stimulated cells; transformation of LTC4 into LTB4-isomers occurs in the presence of stimulated cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. K. Mediators of anaphylaxis and inflammation. Annu Rev Microbiol. 1982;36:371–413. doi: 10.1146/annurev.mi.36.100182.002103. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Hamberg M., Samuelsson B. Transformation of arachidonic acid and homo-gamma-linolenic acid by rabbit polymorphonuclear leukocytes. Monohydroxy acids from novel lipoxygenases. J Biol Chem. 1976 Dec 25;251(24):7816–7820. [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J Biol Chem. 1979 Aug 25;254(16):7865–7869. [PubMed] [Google Scholar]
- Borgeat P., Sirois P. Leukotrienes: a major step in the understanding of immediate hypersensitivity reactions. J Med Chem. 1981 Feb;24(2):121–126. doi: 10.1021/jm00134a001. [DOI] [PubMed] [Google Scholar]
- Bremm K. D., Brom H. J., Alouf J. E., König W., Spur B., Crea A., Peters W. Generation of leukotrienes from human granulocytes by alveolysin from Bacillus alvei. Infect Immun. 1984 Apr;44(1):188–193. doi: 10.1128/iai.44.1.188-193.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brom J., Raulf M., Stüning M., Spur B., Crea A., Bremm K. D., König W. Subcellular localization of enzymes involved in leukotriene formation within human polymorphonuclear granulocytes. Immunology. 1984 Mar;51(3):571–583. [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Claesson H. E., Lundberg U., Malmsten C. Serum-coated zymosan stimulates the synthesis of leukotriene B4 in human polymorphonuclear leukocytes. Inhibition by cyclic AMP. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1230–1237. doi: 10.1016/0006-291x(81)90751-8. [DOI] [PubMed] [Google Scholar]
- Czarnetzki B. M., König W., Lichtenstein L. M. Eosinophil chemotactic factor (ECF). I. Release from polymorphonuclear leukocytes by the calcium ionophore A23187. J Immunol. 1976 Jul;117(1):229–234. [PubMed] [Google Scholar]
- Goetzl E. J. The conversion of leukotriene C4 to isomers of leukotriene B4 by human eosinophil peroxidase. Biochem Biophys Res Commun. 1982 May 31;106(2):270–275. doi: 10.1016/0006-291x(82)91105-6. [DOI] [PubMed] [Google Scholar]
- Henderson W. R., Jörg A., Klebanoff S. J. Eosinophil peroxidase-mediated inactivation of leukotrienes B4, C4, and D4. J Immunol. 1982 Jun;128(6):2609–2613. [PubMed] [Google Scholar]
- Henderson W. R., Klebanoff S. J. Leukotriene B4, C4, D4 and E4 inactivation by hydroxyl radicals. Biochem Biophys Res Commun. 1983 Jan 14;110(1):266–272. doi: 10.1016/0006-291x(83)91290-1. [DOI] [PubMed] [Google Scholar]
- Henderson W. R., Klebanoff S. J. Leukotriene production and inactivation by normal, chronic granulomatous disease and myeloperoxidase-deficient neutrophils. J Biol Chem. 1983 Nov 25;258(22):13522–13527. [PubMed] [Google Scholar]
- Jakschik B. A., Harper T., Murphy R. C. Leukotriene C4 and D4 formation by particulate enzymes. J Biol Chem. 1982 May 25;257(10):5346–5349. [PubMed] [Google Scholar]
- Kroegel C., Kunau H. W., König W. Inhibition of the eosinophil chemotactic factor (ECF) release from human PMNs and rat mast cells by arachidonic acid analogs. Cell Immunol. 1981 May 15;60(2):480–488. doi: 10.1016/0008-8749(81)90288-4. [DOI] [PubMed] [Google Scholar]
- Kroegel C., König W., Mollay C., Kreil G. Generation of the eosinophil chemotactic factor (ECF) from various cell types by melittin. Mol Immunol. 1981 Mar;18(3):227–236. doi: 10.1016/0161-5890(81)90089-4. [DOI] [PubMed] [Google Scholar]
- König W., Czarnetzki B. M., Lichtenstein L. M. Eosinophil chemotactic factor (ECF). II. Release from human polymorphonuclear leukocytes during phagocytosis. J Immunol. 1976 Jul;117(1):235–241. [PubMed] [Google Scholar]
- Lane T. A., Lamkin G. E. Myeloperoxidase-mediated modulation of chemotactic peptide binding to human neutrophils. Blood. 1983 Jun;61(6):1203–1207. [PubMed] [Google Scholar]
- Lee C. W., Lewis R. A., Corey E. J., Barton A., Oh H., Tauber A. I., Austen K. F. Oxidative inactivation of leukotriene C4 by stimulated human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4166–4170. doi: 10.1073/pnas.79.13.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. W., Lewis R. A., Tauber A. I., Mehrotra M., Corey E. J., Austen K. F. The myeloperoxidase-dependent metabolism of leukotrienes C4, D4, and E4 to 6-trans-leukotriene B4 diastereoisomers and the subclass-specific S-diastereoisomeric sulfoxides. J Biol Chem. 1983 Dec 25;258(24):15004–15010. [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. Mediation of local homeostasis and inflammation by leukotrienes and other mast cell-dependent compounds. Nature. 1981 Sep 10;293(5828):103–108. doi: 10.1038/293103a0. [DOI] [PubMed] [Google Scholar]
- Markert M., Allaz M. J., Frei J. Continuous monitoring of oxygen consumption and superoxide production by particle-stimulated human polymorphonuclear leukocytes. FEBS Lett. 1980 May 5;113(2):225–230. doi: 10.1016/0014-5793(80)80597-7. [DOI] [PubMed] [Google Scholar]
- Neill M. A., Henderson W. R., Klebanoff S. J. Oxidative degradation of leukotriene C4 by human monocytes and monocyte-derived macrophages. J Exp Med. 1985 Nov 1;162(5):1634–1644. doi: 10.1084/jem.162.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. S. Properties of leukotriene B4 20-hydroxylase from polymorphonuclear leukocytes. J Biol Chem. 1984 Mar 10;259(5):3082–3089. [PubMed] [Google Scholar]
- Raulf M., Stüning M., König W. Metabolism of leukotrienes by L-gamma-glutamyl-transpeptidase and dipeptidase from human polymorphonuclear granulocytes. Immunology. 1985 May;55(1):135–147. [PMC free article] [PubMed] [Google Scholar]
- Rådmark O., Malmsten C., Samuelsson B., Clark D. A., Goto G., Marfat A., Corey E. J. Leukotriene A: stereochemistry and enzymatic conversion to leukotriene B. Biochem Biophys Res Commun. 1980 Feb 12;92(3):954–961. doi: 10.1016/0006-291x(80)90795-0. [DOI] [PubMed] [Google Scholar]
- Rådmark O., Shimizu T., Jörnvall H., Samuelsson B. Leukotriene A4 hydrolase in human leukocytes. Purification and properties. J Biol Chem. 1984 Oct 25;259(20):12339–12345. [PubMed] [Google Scholar]
- Shak S., Goldstein I. M. Omega-oxidation is the major pathway for the catabolism of leukotriene B4 in human polymorphonuclear leukocytes. J Biol Chem. 1984 Aug 25;259(16):10181–10187. [PubMed] [Google Scholar]
- Weller P. F., Lee C. W., Foster D. W., Corey E. J., Austen K. F., Lewis R. A. Generation and metabolism of 5-lipoxygenase pathway leukotrienes by human eosinophils: predominant production of leukotriene C4. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7626–7630. doi: 10.1073/pnas.80.24.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. J., Cole P. J. Polymorphonuclear leucocyte membrane-stimulated oxidative metabolic activity---the effect of divalent cations and cytochalasins. Immunology. 1981 Dec;44(4):847–858. [PMC free article] [PubMed] [Google Scholar]
- Williams J. D., Lee T. H., Lewis R. A., Austen F. Intracellular retention of the 5-lipoxygenase pathway product, leukotriene B4, by human neutrophils activated with unopsonized zymosan. J Immunol. 1985 Apr;134(4):2624–2630. [PubMed] [Google Scholar]


