Abstract
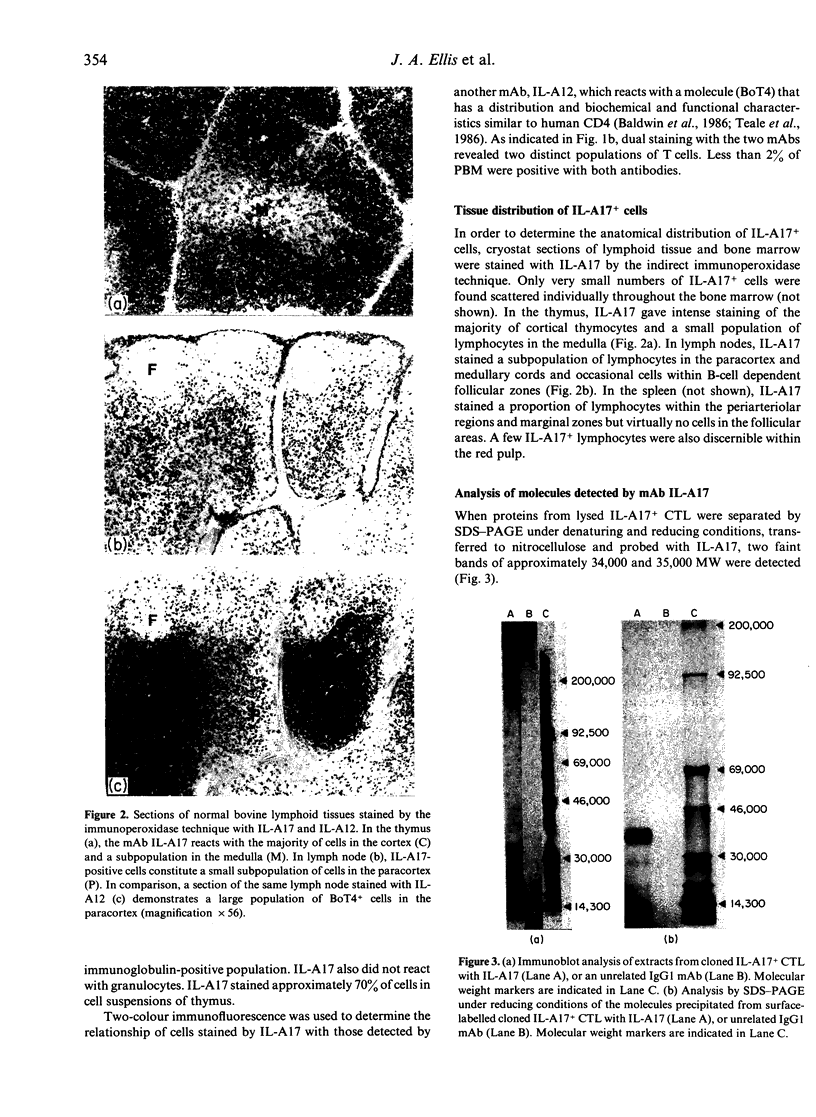
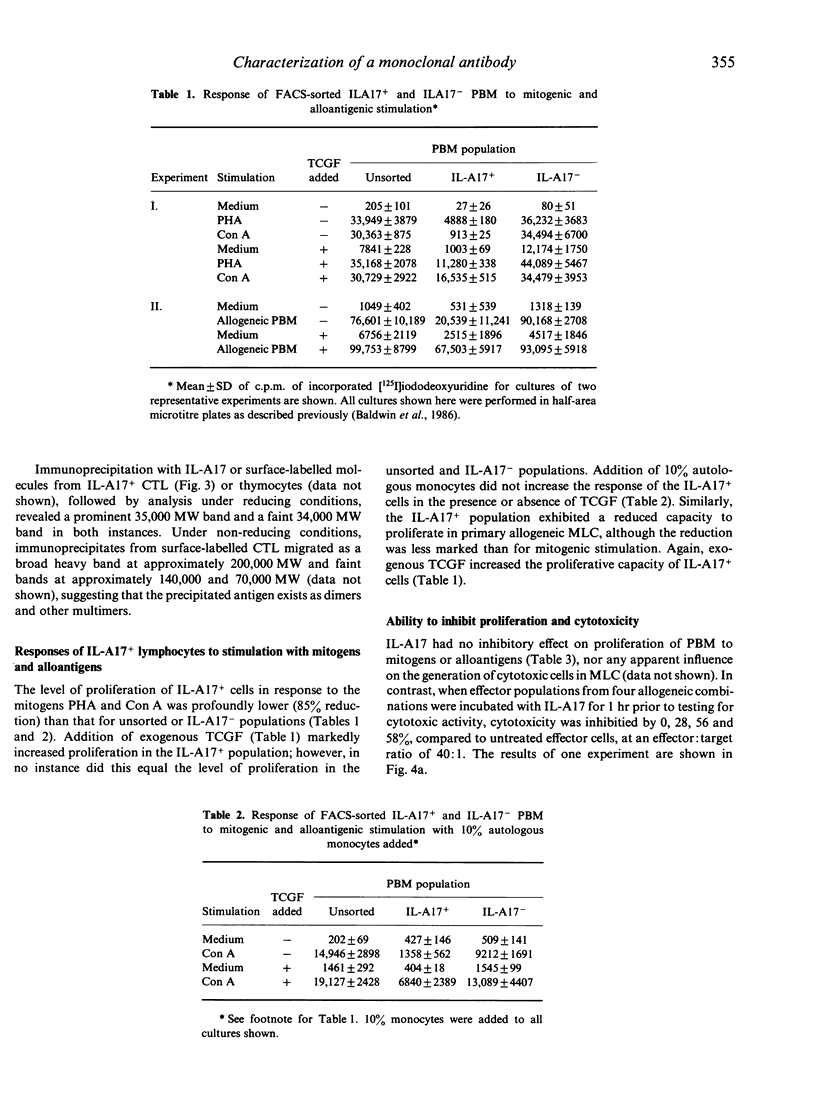
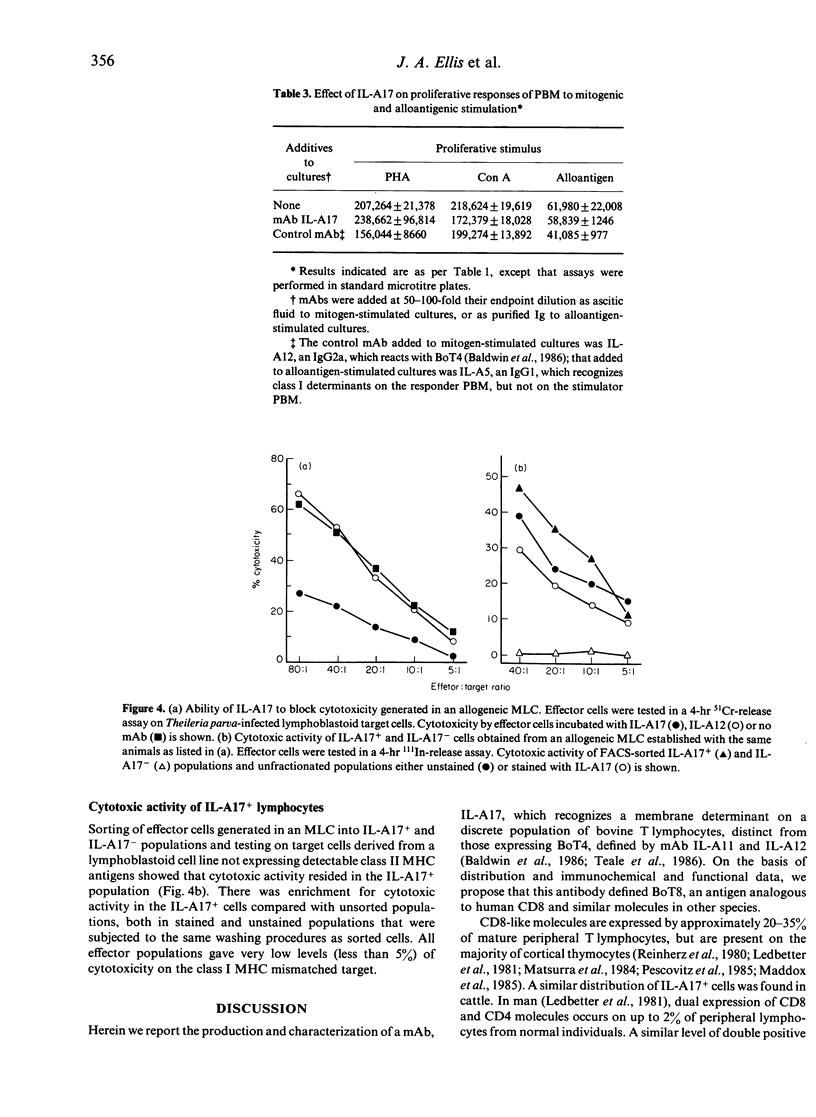
Monoclonal antibody (mAb) IL-A17 characterizes a subset of bovine T lymphocytes. IL-A17 recognizes a 34,000-35,000 MW doublet, designated BoT8, which is expressed on the surface of approximately 20% of peripheral blood mononuclear leucocytes (PBM), a subpopulation of lymphocytes in T-dependent areas of lymph nodes and spleen, and about 70% of thymocytes. This molecule is not expressed on B lymphocytes, monocytes/macrophages or granulocytes. Double labelling of PBM showed that the BoT8+ population is distinct from the T lymphocyte subset expressing BoT4. BoT8+ lymphocytes purified with a fluorescence-activated cell sorter (FACS) proliferated poorly in response to mitogenic and alloantigenic stimulation in the absence of exogenous growth factors. IL-A17 had no inhibitory effect on proliferation of PBM to mitogens (Con A and PHA) or alloantigens and no measurable effect on the in vitro generation of cytolytic effector cells. However, in some experiments IL-A17 was found to block partially allospecific cytolytic function mediated by bulk effector cell populations when included in 51Cr-release assays. Fractionation of effector cells generated in an allogeneic mixed leucocyte culture (MLC) demonstrated that cytotoxic cells specific for class I major histocompatibility complex (MHC) antigens reside within the BoT8+ population. Based on these data, and information reported elsewhere on alloreactive bovine T-cell clones, BoT8 is considered to be analogous to CD8 in humans and equivalent molecules in other species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Hollander N. Effects of anti-Lyt antibodies on T-cell functions. Immunol Rev. 1982;68:43–66. doi: 10.1111/j.1600-065x.1982.tb01059.x. [DOI] [PubMed] [Google Scholar]
- Jandinski J., Cantor H., Tadakuma T., Peavy D. L., Pierce C. W. Separation of helper T cells from suppressor T cells expressing different Ly components. I. Polyclonal activation: suppressor and helper activities are inherent properties of distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1382–1390. doi: 10.1084/jem.143.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonjić S., Koszinowski U. H. Monoclonal antibodies reactive with swine lymphocytes. I. Antibodies to membrane structures that define the cytolytic T lymphocyte subset in the swine. J Immunol. 1984 Aug;133(2):647–652. [PubMed] [Google Scholar]
- Lanier L. L., Engleman E. G., Gatenby P., Babcock G. F., Warner N. L., Herzenberg L. A. Correlation of functional properties of human lymphoid cell subsets and surface marker phenotypes using multiparameter analysis and flow cytometry. Immunol Rev. 1983;74:143–160. doi: 10.1111/j.1600-065x.1983.tb01088.x. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Glasebrook A. L., Cerottini J. C. Clonal heterogeneity in the functional requirement for Lyt-2/3 molecules on cytolytic T lymphocytes: analysis by antibody blocking and selective trypsinization. J Exp Med. 1982 Dec 1;156(6):1711–1722. doi: 10.1084/jem.156.6.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. Surface antigens, SBU-T4 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology. 1985 Aug;55(4):739–748. [PMC free article] [PubMed] [Google Scholar]
- Matsuura A., Ishii Y., Yuasa H., Narita H., Kon S., Takami T., Kikuchi K. Rat T lymphocyte antigens comparable with mouse Lyt-1 and Lyt-2,3 antigenic systems: characterization by monoclonal antibodies. J Immunol. 1984 Jan;132(1):316–322. [PubMed] [Google Scholar]
- Meuer S. C., Schlossman S. F., Reinherz E. L. Clonal analysis of human cytotoxic T lymphocytes: T4+ and T8+ effector T cells recognize products of different major histocompatibility complex regions. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4395–4399. doi: 10.1073/pnas.79.14.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T. W., Pinder M., Roelants G. E., Kar S. K., Lundin L. B., Mayor-Withey K. S., Hwett R. S. Methods for derivation and detection of anti-parasite monoclonal antibodies. J Immunol Methods. 1980;34(2):141–154. doi: 10.1016/0022-1759(80)90168-4. [DOI] [PubMed] [Google Scholar]
- Pescovitz M. D., Lunney J. K., Sachs D. H. Murine anti-swine T4 and T8 monoclonal antibodies: distribution and effects on proliferative and cytotoxic T cells. J Immunol. 1985 Jan;134(1):37–44. [PubMed] [Google Scholar]
- Pescovitz M. D., Lunney J. K., Sachs D. H. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol. 1984 Jul;133(1):368–375. [PubMed] [Google Scholar]
- Platsoucas C. D. Human T cell antigens involved in cytotoxicity against allogeneic or autologous chemically modified targets. Association of the Leu 2a/T8 antigen with effector-target cell binding and of the T3/Leu 4 antigen with triggering. Eur J Immunol. 1984 Jun;14(6):566–577. doi: 10.1002/eji.1830140615. [DOI] [PubMed] [Google Scholar]
- Poppema S., Bhan A. K., Reinherz E. L., McCluskey R. T., Schlossman S. F. Distribution of T cell subsets in human lymph nodes. J Exp Med. 1981 Jan 1;153(1):30–41. doi: 10.1084/jem.153.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Wilson A. A new assay for cytotoxic lymphocytes, based on a radioautographic readout of 111 In release, suitable for rapid, semi-automated assessment of limit-dilution cultures. J Immunol Methods. 1981;43(2):135–152. doi: 10.1016/0022-1759(81)90017-x. [DOI] [PubMed] [Google Scholar]
- Snow P. M., Terhorst C. The T8 antigen is a multimeric complex of two distinct subunits on human thymocytes but consists of homomultimeric forms on peripheral blood T lymphocytes. J Biol Chem. 1983 Dec 10;258(23):14675–14681. [PubMed] [Google Scholar]
- Straus W. Imidazole increases the sensitivity of the cytochemical reaction for peroxidase with diaminobenzidine at a neutral pH. J Histochem Cytochem. 1982 May;30(5):491–493. doi: 10.1177/30.5.6176617. [DOI] [PubMed] [Google Scholar]
- Swain S. L. T cell subsets and the recognition of MHC class. Immunol Rev. 1983;74:129–142. doi: 10.1111/j.1600-065x.1983.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Teale A. J., Morrison W. I., Goddeeris B. M., Groocock C. M., Stagg D. A., Spooner R. L. Bovine alloreactive cytotoxic cells generated in vitro: target specificity in relation to BoLA phenotype. Immunology. 1985 Jun;55(2):355–362. [PMC free article] [PubMed] [Google Scholar]
- van Ewijk W., van Soest P. L., van den Engh G. J. Fluorescence analysis and anatomic distribution of mouse T lymphocyte subsets defined by monoclonal antibodies to the antigens Thy-1, Lyt-1, Lyt-2, and T-200. J Immunol. 1981 Dec;127(6):2594–2604. [PubMed] [Google Scholar]





