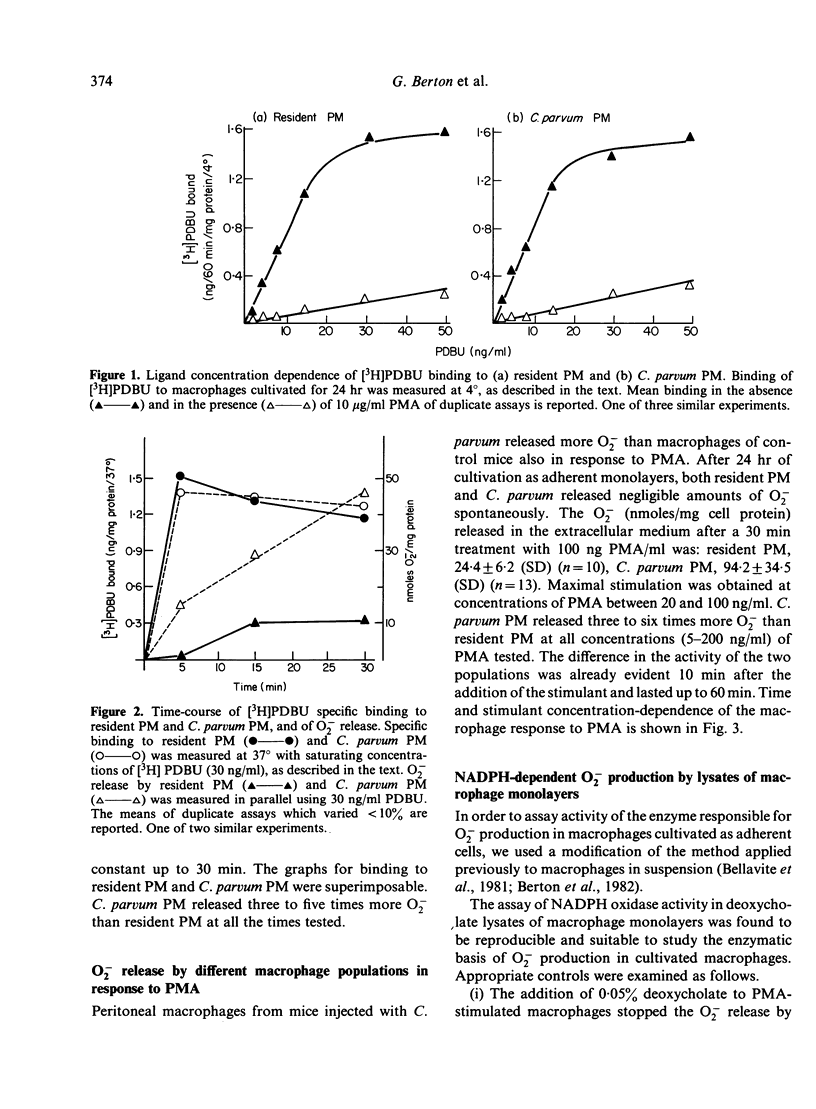
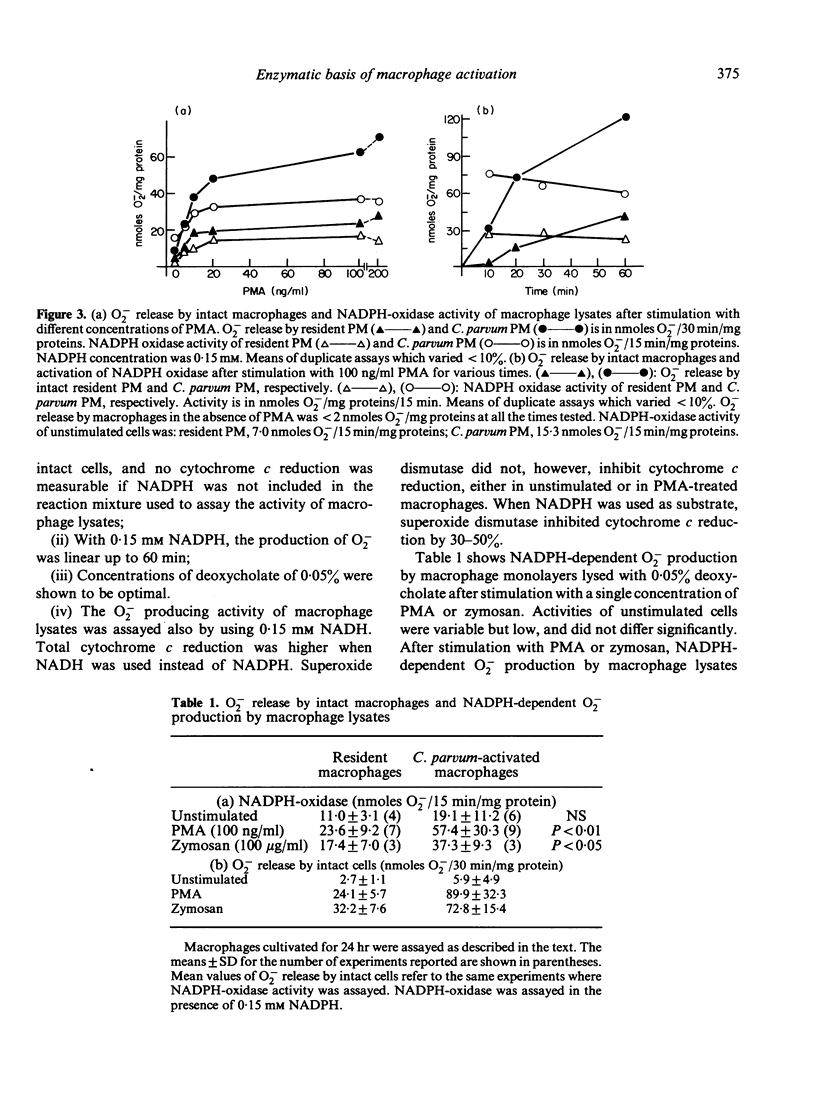
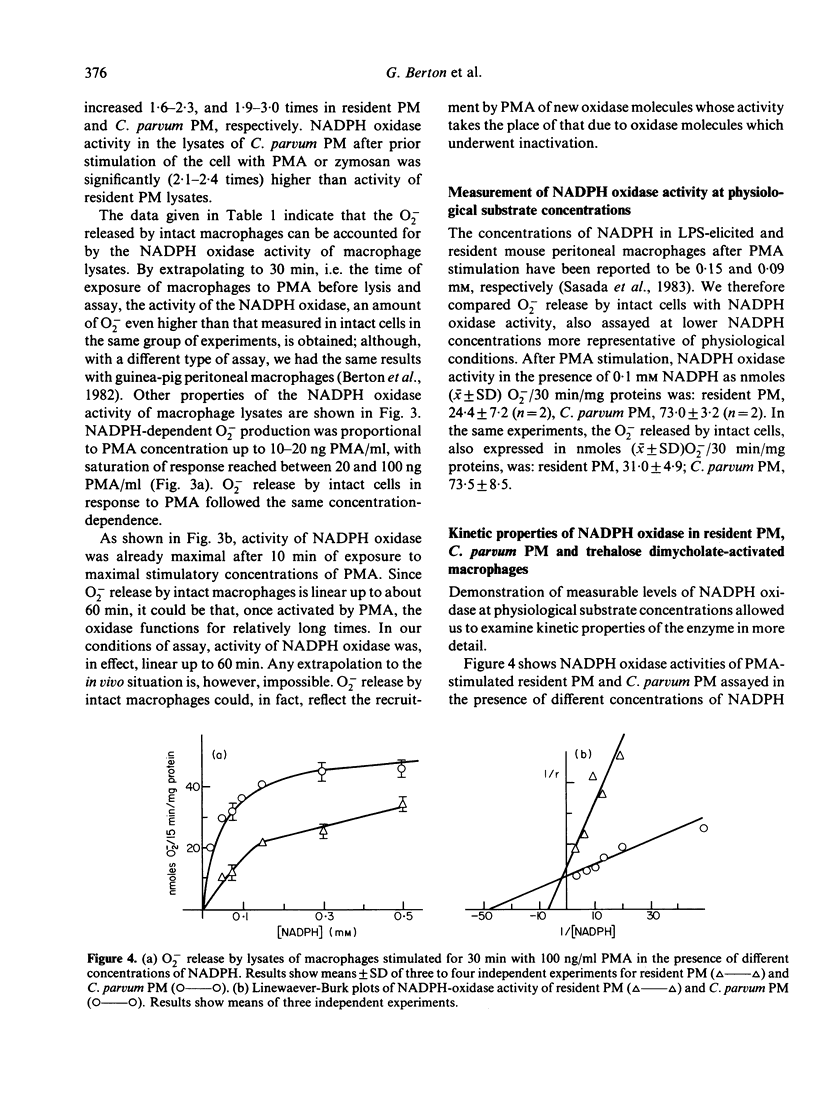
Abstract
Mouse peritoneal macrophages activated in vivo by the injection of Corynebacterium parvum release larger amounts of superoxide anion (O2-) than macrophages from control mice when stimulated with phorbol myristate acetate (PMA). The biochemical bases for this enhanced response of activated macrophages have been investigated by studying the expression and function of receptors for the stimulant, and the activity of the enzyme NADPH oxidase which is responsible for the production of O2- in leucocytes. Studies of binding of phorbol dibutyrate, an agent closely related to PMA, showed that the affinity constants (Kds) and the number of binding sites were the same in resident and activated peritoneal macrophages. The activity of the NADPH oxidase was, however, different in the two macrophage populations which differ in their capacity to release O2-. NADPH oxidase activity was studied in macrophage monolayers after lysis with deoxycholate. The main features of this activity were as follows: stimulation of macrophages with PMA or zymosan caused an increase in NADPH-dependent O2- production; NADPH oxidase activity in the lysates followed the same dose-response curve for different concentrations of PMA as O2- release by intact macrophages; O2- release by intact macrophages could be fully accounted for by NADPH-dependent O2- production by macrophage lysates; activity was strictly substrate-specific, in that NADH could not substitute for NADPH; after stimulation with PMA or zymosan, NADPH oxidase activity was higher in lysates of C. parvum-activated macrophages than in lysates of resident macrophages; NADPH oxidase activities of activated and resident macrophages differed markedly in their kinetic parameters. The NADPH oxidase of macrophages activated by C. parvum or trehalose dimycolate of mycobacterial origin displayed a five to seven times lower Km compared to the enzyme in resident macrophages.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavite P., Berton G., Dri P., Soranzo M. R. Enzymatic basis of the respiratory burst of guinea pig resident peritoneal macrophages. J Reticuloendothel Soc. 1981 Jan;29(1):47–60. [PubMed] [Google Scholar]
- Bellavite P., Dri P., Della Bianca V., Serra M. C. The measurement of superoxide anion production by granulocytes In whole blood. A clinical test for the evaluation of phagocyte function and serum opsonic capacity. Eur J Clin Invest. 1983 Aug;13(4):363–368. doi: 10.1111/j.1365-2362.1983.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Berton G., Bellavite P., Dri P., de Togni P., Rossi F. The enzyme responsible for the respiratory burst in elicited guinea pig peritoneal macrophages. J Pathol. 1982 Apr;136(4):273–290. doi: 10.1002/path.1711360403. [DOI] [PubMed] [Google Scholar]
- Berton G., Gordon S. Desensitization of macrophages to stimuli which induce secretion of superoxide anion. Down-regulation of receptors for phorbol myristate acetate. Eur J Immunol. 1983 Aug;13(8):620–627. doi: 10.1002/eji.1830130804. [DOI] [PubMed] [Google Scholar]
- Berton G., Gordon S. Superoxide release by peritoneal and bone marrow-derived mouse macrophages. Modulation by adherence and cell activation. Immunology. 1983 Aug;49(4):693–704. [PMC free article] [PubMed] [Google Scholar]
- Cummings N. P., Pabst M. J., Johnston R. B., Jr Activation of macrophages for enhanced release of superoxide anion and greater killing of Candida albicans by injection of muramyl dipeptide. J Exp Med. 1980 Dec 1;152(6):1659–1669. doi: 10.1084/jem.152.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Gordon S. Down-regulation of mannosyl receptor-mediated endocytosis and antigen F4/80 in bacillus Calmette-Guérin-activated mouse macrophages. Role of T lymphocytes and lymphokines. J Exp Med. 1982 Jun 1;155(6):1623–1637. doi: 10.1084/jem.155.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Chadwick D. A., Cohn Z. A. Priming of macrophages for enhanced oxidative metabolism by exposure to proteolytic enzymes. J Exp Med. 1981 Jun 1;153(6):1678–1683. doi: 10.1084/jem.153.6.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepoivre M., Tenu J. P., Lemaire G., Petit J. F. Antitumor activity and hydrogen peroxide release by macrophages elicited by trehalose diesters. J Immunol. 1982 Aug;129(2):860–866. [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980 Dec 1;152(6):1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Root R. K. Hydrogen peroxide release from mouse peritoneal macrophages: dependence on sequential activation and triggering. J Exp Med. 1977 Dec 1;146(6):1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Romeo D., Zabucchi G., Marzi T., Rossi F. Kinetic and enzymatic features of metabolic stimulation of alveolar and peritoneal macrophages challenged with bacteria. Exp Cell Res. 1973 Apr;78(2):423–432. doi: 10.1016/0014-4827(73)90087-6. [DOI] [PubMed] [Google Scholar]
- Sasada M., Pabst M. J., Johnston R. B., Jr Activation of mouse peritoneal macrophages by lipopolysaccharide alters the kinetic parameters of the superoxide-producing NADPH oxidase. J Biol Chem. 1983 Aug 25;258(16):9631–9635. [PubMed] [Google Scholar]
- Tsunawaki S., Nathan C. F. Enzymatic basis of macrophage activation. Kinetic analysis of superoxide production in lysates of resident and activated mouse peritoneal macrophages and granulocytes. J Biol Chem. 1984 Apr 10;259(7):4305–4312. [PubMed] [Google Scholar]
- Weinberg J. B., Misukonis M. A. Phorbol diester-induced H2O2 production by peritoneal macrophages. Different H2O2 production by macrophages from normal and BCG-infected mice despite comparable phorbol diester receptors. Cell Immunol. 1983 Sep;80(2):405–415. doi: 10.1016/0008-8749(83)90127-2. [DOI] [PubMed] [Google Scholar]


