Abstract
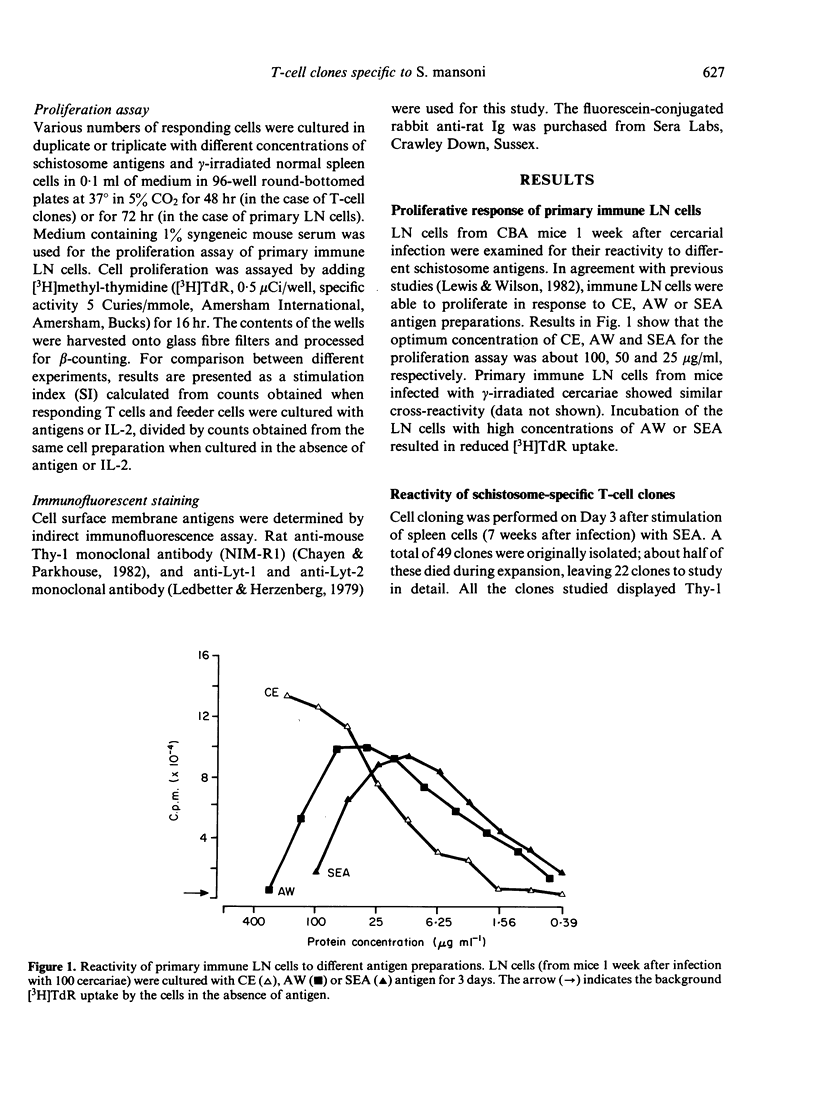
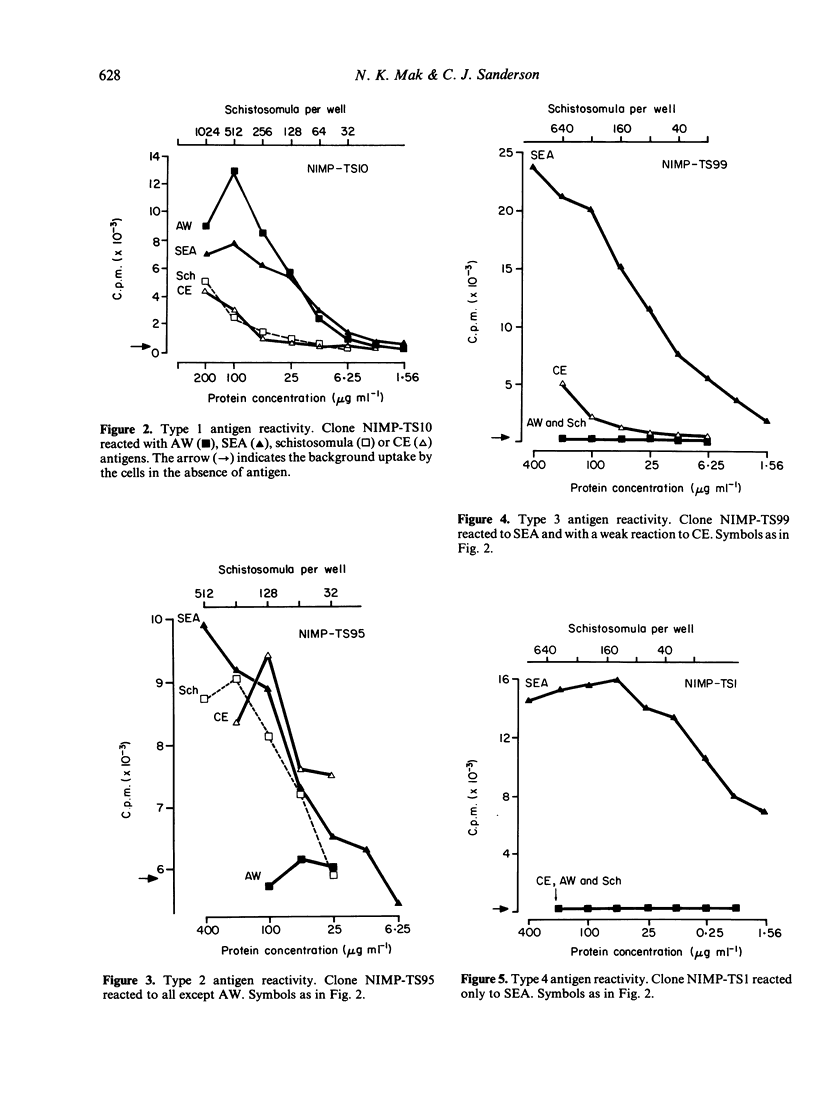
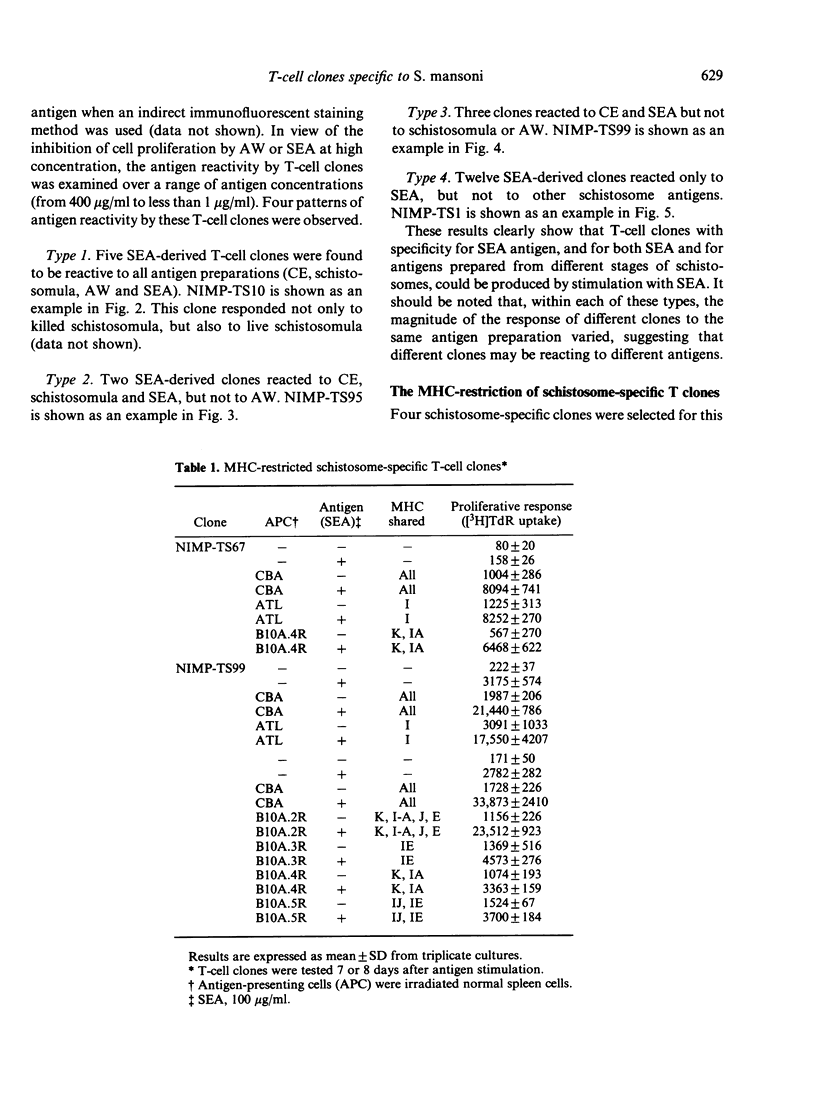
A panel of T-cell clones reactive to the soluble egg antigen (SEA) of Schistosoma mansoni is described. The proliferative responses of the primary immune lymph node (LN) cells and T-cell clones to different schistosome antigen preparations (cercarial extract (CE), live or dead schistosomula, adult worm extract, and soluble egg antigen) were compared. Primary immune LN cells could not distinguish between these schistosome antigen preparations. However, when a total of 22 T-cell clones was analysed, a complex pattern of both stage-specific and common (or cross-reactive) antigen reactivity was observed. These patterns have been grouped into four types: (i) five clones were reactive to all different schistosome antigen preparations; (ii) two clones were reactive only to SEA, CE and schistosomula; (iii) three clones were reactive only to SEA and cercariae; (iv) twelve clones were reactive to SEA antigen alone. The T-cell clones were identified as Thy-1-positive cells. All the clones were able to respond to exogenous IL-2 after antigenic stimulation. However, there was a variable degree of IL-2 responsiveness when compared with antigen-specific stimulation. Four T clones were selected for further studies on the genetic control of the proliferative response to schistosome antigens. One of the proliferative T clones was restricted by the IA subregion of the major histocompatibility complex (MHC), and the other three clones are restricted by the IE (AeE alpha) subregion.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Leung K. N., Ertl H. An analysis of effector T cell generation and function in mice exposed to influenza A or Sendai viruses. Immunol Rev. 1981;58:5–24. doi: 10.1111/j.1600-065x.1981.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Andrew M. E., Braciale V. L., Braciale T. J. Regulation of interleukin 2 receptor expression on murine cytotoxic T lymphocyte clones. J Immunol. 1984 Feb;132(2):839–844. [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayen A., Parkhouse R. M. Preparation and properties of a cytotoxic monoclonal rat anti-mouse Thy-1 antibody. J Immunol Methods. 1982;49(1):17–23. doi: 10.1016/0022-1759(82)90362-3. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L., David C. S. Regulation of granulomatous inflammation in murine schistosomiasis. II. T suppressor cell-derived, I-C subregion-encoded soluble suppressor factor mediates regulation of lymphokine production. J Exp Med. 1983 Jan 1;157(1):219–230. doi: 10.1084/jem.157.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Wellhausen S. R., Boros D. L. Modulation of granulomatous hypersensitivity. II. Participation of Ly 1+ and Ly 2+ T lymphocytes in the suppression of granuloma formation and lymphokine production in Schistosoma mansoni-infected mice. J Immunol. 1981 Jul;127(1):363–367. [PubMed] [Google Scholar]
- Cioli D., Dennert G. The course of Schistosoma mansoni infection in thymectomized rats. J Immunol. 1976 Jul;117(1):59–65. [PubMed] [Google Scholar]
- Conrad P. J., Janeway C. A., Jr The expression of I-Ed molecules in F1 hybrid mice detected with antigen-specific, I-Ed-restricted cloned T-cell lines. Immunogenetics. 1984;20(3):311–319. doi: 10.1007/BF00364212. [DOI] [PubMed] [Google Scholar]
- Damian R. T. Immunity in schistosomiasis: a holistic view. Contemp Top Immunobiol. 1984;12:359–420. doi: 10.1007/978-1-4684-4571-8_10. [DOI] [PubMed] [Google Scholar]
- Doenhoff M., Long E. Factors affecting the acquisition of resistance against Schistosoma mansoni in the mouse. IV. The inability of T-cell-deprived mice to resist re-infection, and other in vivo studies on the mechanisms of resistance. Parasitology. 1979 Apr;78(2):171–183. doi: 10.1017/s0031182000049222. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Fuller-Farrar J., Simon P. L., Hilfiker M. L., Stadler B. M., Farrar W. L. Thymoma production of T cell growth factor (Interleukin 2). J Immunol. 1980 Dec;125(6):2555–2558. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Hemler M. E., Brenner M. B., McLean J. M., Strominger J. L. Antigenic stimulation regulates the level of expression of interleukin 2 receptor on human T cells. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2172–2175. doi: 10.1073/pnas.81.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. L. In vitro proliferative response to living schistosomula by T lymphocytes from mice infected with Schistosoma mansoni. Parasitology. 1981 Aug;83(Pt 1):147–162. doi: 10.1017/s0031182000050125. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Braciale V. L., Braciale T. J. Antigen-dependent regulation of interleukin 2 receptor expression on cloned human cytotoxic T lymphocytes. J Immunol. 1984 Oct;133(4):1966–1969. [PubMed] [Google Scholar]
- Krco C. J., Wassom D. L., Abramson E. J., David C. S. Cloned T cells recognize Trichinella spiralis antigen in association with an Ek beta Ek alpha restriction element. Immunogenetics. 1983;18(5):435–444. doi: 10.1007/BF00364385. [DOI] [PubMed] [Google Scholar]
- Larsson E. L. Mechanism of T cell activation. II. Antigen- and lectin-dependent acquisition of responsiveness to TCGF is a nonmitogenic, active response of resting T cells. J Immunol. 1981 Apr;126(4):1323–1326. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lewis F. A., Wilson E. M. Regional and splenic lymphocyte proliferative responses of mice exposed to normal or irradiated Schistosoma mansoni cercariae. Am J Trop Med Hyg. 1982 May;31(3 Pt 1):505–513. doi: 10.4269/ajtmh.1982.31.505. [DOI] [PubMed] [Google Scholar]
- Louis J. A., Lima G., Pestel J., Titus R. Murine T-cell responses to protozoan and metazoan parasites: functional analysis of T-cell lines and clones specific for Leishmania tropica and Schistosoma mansoni. Contemp Top Immunobiol. 1984;12:201–224. doi: 10.1007/978-1-4684-4571-8_6. [DOI] [PubMed] [Google Scholar]
- Marusić M., Nagy Z. A., Koszinowski U., Klein J. Involvement of Mhc loci in immune responses that are not Ir-gene-controlled. Immunogenetics. 1982;16(5):471–483. doi: 10.1007/BF00372105. [DOI] [PubMed] [Google Scholar]
- Matis L. A., Jones P. P., Murphy D. B., Hedrick S. M., Lerner E. A., Janeway C. A., Jr, McNicholas J. M., Schwartz R. H. Immune response gene function correlates with the expression of an Ia antigen. II. A quantitative deficiency in Ae:E alpha complex expression causes a corresponding defect in antigen-presenting cell function. J Exp Med. 1982 Feb 1;155(2):508–523. doi: 10.1084/jem.155.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Cruise K. M., Garcia E. G., Anders R. F. Hybridoma-derived antibody with immunodiagnostic potential for schistosomiasis japonica. Proc Natl Acad Sci U S A. 1981 May;78(5):3165–3169. doi: 10.1073/pnas.78.5.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z. A., Baxevanis C. N., Ishii N., Klein J. Ia antigens as restriction molecules in Ir-gene controlled T-cell proliferation. Immunol Rev. 1981;60:59–83. doi: 10.1111/j.1600-065x.1981.tb00362.x. [DOI] [PubMed] [Google Scholar]
- Phillips S. M., Bentley A. G., Linette G., Doughty B. L., Capron M. The immunologic response of congenitally athymic rats to Schistosoma mansoni infection. I. In vivo studies of resistance. J Immunol. 1983 Sep;131(3):1466–1474. [PubMed] [Google Scholar]
- Phillips S. M., DiConza J. J., Gold J. A., Reid W. A. Schistosomiasis in the congenitally athymic (nude) mouse. I. Thymic dependency of eosinophilia, granuloma formation, and host morbidity. J Immunol. 1977 Feb;118(2):594–599. [PubMed] [Google Scholar]
- Ramalho-Pinto F. J., Gazzinelli G., Howells R. E., Mota-Santos T. A., Figueiredo E. A., Pellegrino J. Schistosoma mansoni: defined system for stepwise transformation of cercaria to schistosomule in vitro. Exp Parasitol. 1974 Dec;36(3):360–372. doi: 10.1016/0014-4894(74)90076-9. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., Chen C., Paul W. E. Gene complementation in the T lymphocyte proliferative response to poly (Glu56Lys35Phe9)n. Functional evidence for a restriction element coded for by both the I-A and I-E subregions. Eur J Immunol. 1980 Sep;10(9):708–714. doi: 10.1002/eji.1830100910. [DOI] [PubMed] [Google Scholar]
- Sher A., Hieny S., James S. L., Asofsky R. Mechanisms of protective immunity against Schistosoma mansoni infection in mice vaccinated with irradiated cercariae. II. Analysis of immunity in hosts deficient in T lymphocytes, B lymphocytes, or complement. J Immunol. 1982 Apr;128(4):1880–1884. [PubMed] [Google Scholar]
- Sher A., Hieny S., James S. Mechanisms of protective immunity against S. mansoni infection in mice vaccinated with irradiated cercariae. VI. Influence of the major histocompatibility complex. Parasite Immunol. 1984 Jul;6(4):319–328. doi: 10.1111/j.1365-3024.1984.tb00804.x. [DOI] [PubMed] [Google Scholar]
- Simon M. M., Weltzien H. U., Bühring H. J., Eichmann K. Aged murine killer T-cell clones acquire specific cytotoxicity for P815 mastocytoma cells. Nature. 1984 Mar 22;308(5957):367–370. doi: 10.1038/308367a0. [DOI] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Vitiello A., Sherman L. A. Recognition of influenza-infected cells by cytolytic T lymphocyte clones: determinant selection by class I restriction elements. J Immunol. 1983 Oct;131(4):1635–1640. [PubMed] [Google Scholar]
- Weinstock J. V., Boros D. L. Modulation of granulomatous hypersensitivity. VI. T lymphocyte subsets influence mast cell density in liver granulomas of Schistosoma mansoni-infected mice. J Immunol. 1983 Aug;131(2):959–961. [PubMed] [Google Scholar]


