Abstract
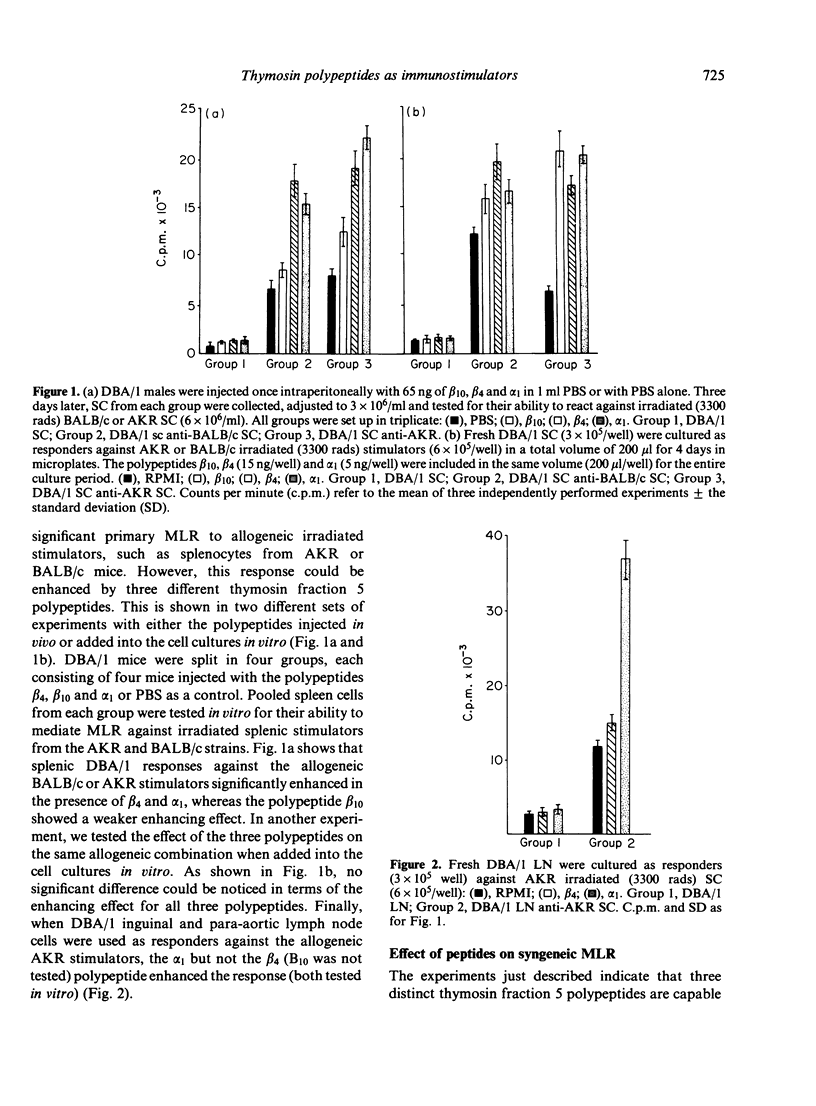
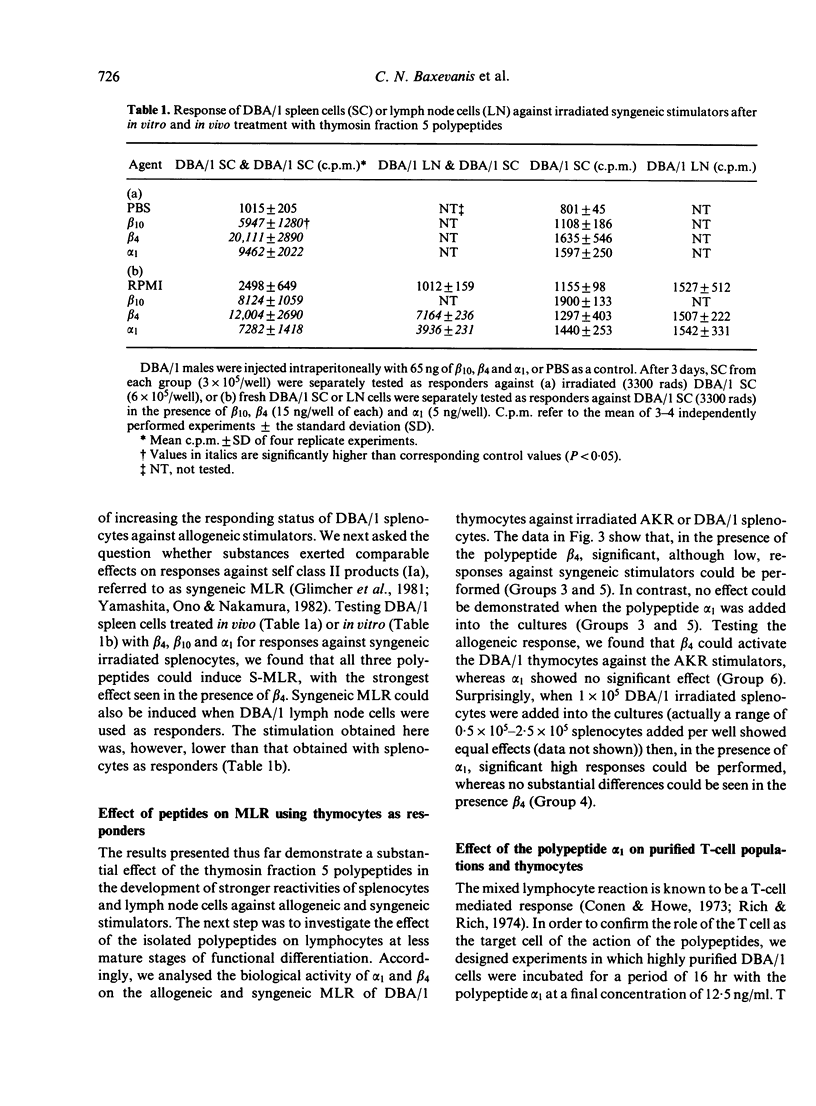
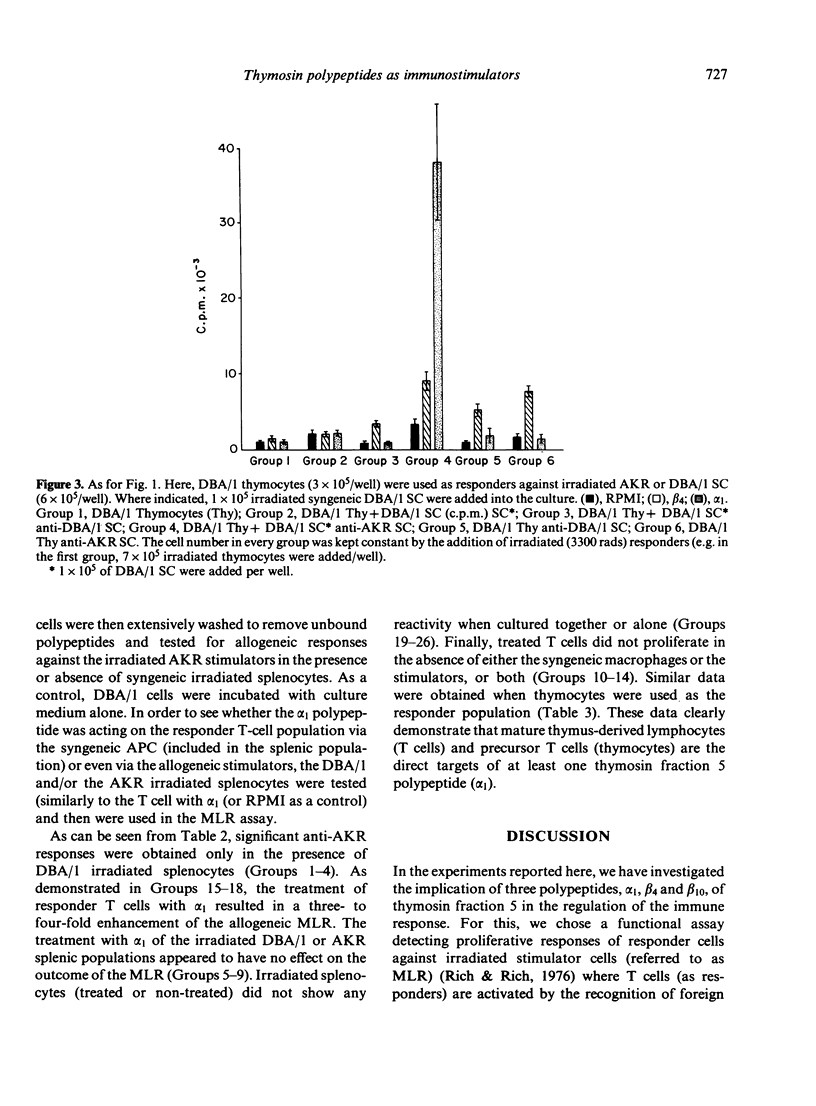
The biological effects of three thymosin fraction 5 polypeptides, designated as beta 10, beta 4, and alpha 1 were tested in the MLR of mouse splenocytes, lymph node cells and thymocytes against syngeneic or allogeneic stimulators. It was found that all three polypeptides, after in vivo and in vitro treatment of the responder cell population, could enhance the allogeneic MLR. These polypeptides were also able to induce significant syngeneic MLR in systems where responder cells were used against irradiated syngeneic splenocytes. In addition, while beta 4 was shown to have a weak stimulatory effect on allogeneic MLR utilizing thymocytes as the responder cell type, alpha 1 could strongly induce such responses when syngeneic splenocytes were included into the culture system. Preincubation of purified mature T cells or thymocytes with alpha 1 has shown these cells to be the target of this polypeptide action. Thus, it appears that thymosin fraction 5 polypeptides not only initiate differentiation processes of immature T cells, but also exert their effects on mature T lymphocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A., Wong D. M., Thurman G. B., Low T. L., Goldstein A. L., Sharkis S. J., Goldschneider I. T-lymphocyte maturation: cell surface markers and immune function induced by T-lymphocyte cell-free products and thymosin polypeptides. Ann N Y Acad Sci. 1979;332:81–94. doi: 10.1111/j.1749-6632.1979.tb47100.x. [DOI] [PubMed] [Google Scholar]
- Armerding D., Katz D. H. Activation of T and B lymphocytes in vitro. IV. Regulatory influence on specific T cell functions by a thymus extract factor. J Immunol. 1975 Apr;114(4):1248–1254. [PubMed] [Google Scholar]
- Bach J. F., Dardenne M., Goldstein A. L., Guha A., White A. Appearance of T-cell markers in bone marrow rosette-forming cells after incubation with thymosin, a thymic hormone. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2734–2738. doi: 10.1073/pnas.68.11.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxevanis C. N., Ishii N., Nagy Z. A., Klein J. H-2-controlled suppression of T cell response to lactate dehydrogenase B. Characterization of the lactate dehydrogenase B suppressor pathway. J Exp Med. 1982 Sep 1;156(3):822–833. doi: 10.1084/jem.156.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L., Howe M. L. Synergism between subpopulations of thymus-derived cells mediating the proliferative and effector phases of the mixed lymphocyte reaction. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2707–2710. doi: 10.1073/pnas.70.9.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher L. H., Longo D. L., Green I., Schwartz R. H. Murine syngeneic mixed lymphocyte response. I. Target antigens are self Ia molecules. J Exp Med. 1981 Nov 1;154(5):1652–1670. doi: 10.1084/jem.154.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., Guha A., Zatz M. M., Hardy M. A., White A. Purification and biological activity of thymosin, a hormone of the thymus gland. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1800–1803. doi: 10.1073/pnas.69.7.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., Slater F. D., White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin). Proc Natl Acad Sci U S A. 1966 Sep;56(3):1010–1017. doi: 10.1073/pnas.56.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannappel E., Xu G. J., Morgan J., Hempstead J., Horecker B. L. Thymosin beta 4: a ubiquitous peptide in rat and mouse tissues. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2172–2175. doi: 10.1073/pnas.79.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN J. J., GOLDSTEIN A. L., WHITE A. ENHANCEMENT OF IN VIVO INCORPORATION OF LABELED PRECURSORS INTO DNA AND TOTAL PROTEIN OF MOUSE LYMPH NODES AFTER ADMINISTRATION OF THYMIC EXTRACTS. Proc Natl Acad Sci U S A. 1965 Apr;53:812–817. doi: 10.1073/pnas.53.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D. B. Maturational effects of thymic hormones on human helper and suppressor T cells: effects of FTS ('Facteur Thymique Sérique') and thymosin. Clin Exp Immunol. 1980 Mar;39(3):722–727. [PMC free article] [PubMed] [Google Scholar]
- Low T. L., Hu S. K., Goldstein A. L. Complete amino acid sequence of bovine thymosin beta 4: a thymic hormone that induces terminal deoxynucleotidyl transferase activity in thymocyte populations. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1162–1166. doi: 10.1073/pnas.78.2.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low T. L., Thurman G. B., McAdoo M., McClure J., Rossio J. L., Naylor P. H., Goldstein A. L. The chemistry and biology of thymosin. I. Isolation, characterization, and biological activities of thymosin alpha1 and polypeptide beta1 from calf thymus. J Biol Chem. 1979 Feb 10;254(3):981–986. [PubMed] [Google Scholar]
- Mage M. G., McHugh L. L., Rothstein T. L. Mouse lymphocytes with and without surface immunoglobulin: preparative scale separation in polystyrene tissue culture dishes coated with specifically purified anti-immunoglobulin. J Immunol Methods. 1977;15(1):47–56. doi: 10.1016/0022-1759(77)90016-3. [DOI] [PubMed] [Google Scholar]
- Rich S. S., Rich R. R. Regulatory mechanisms in cell-mediated immune responses. I. Regulation of mixed lymphocyte reactions by alloantigen-activated thymus-derived lymphocytes. J Exp Med. 1974 Dec 1;140(6):1588–1603. doi: 10.1084/jem.140.6.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich S. S., Rich R. R. Regulatory mechanisms in cell-mediated immune responses. III. I-region control of suppressor cell interaction with responder cells in mixed lymphocyte reactions. J Exp Med. 1976 Mar 1;143(3):672–677. doi: 10.1084/jem.143.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita U., Ono S., Nakamura H. The syngeneic mixed leukocyte reaction in mice. II. The I region control of suppressor T cell activity induced in the syngeneic mixed leukocyte reaction. J Immunol. 1982 Mar;128(3):1010–1017. [PubMed] [Google Scholar]


