Abstract
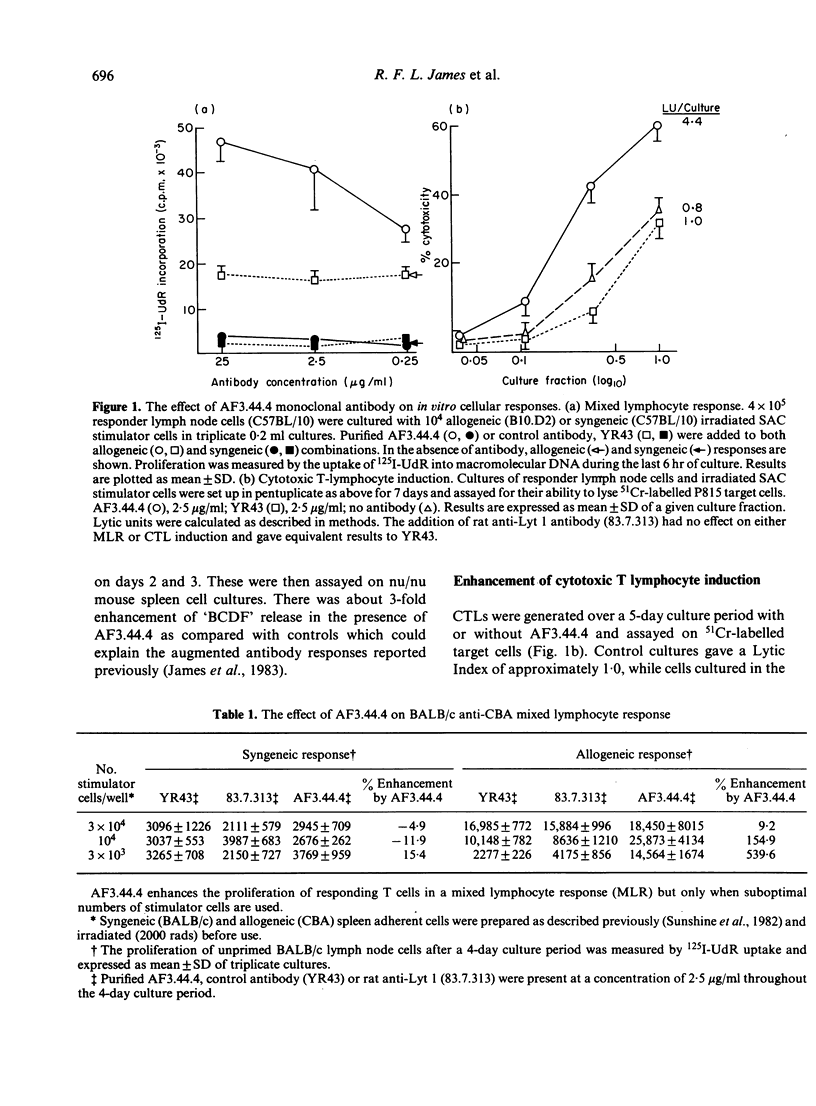
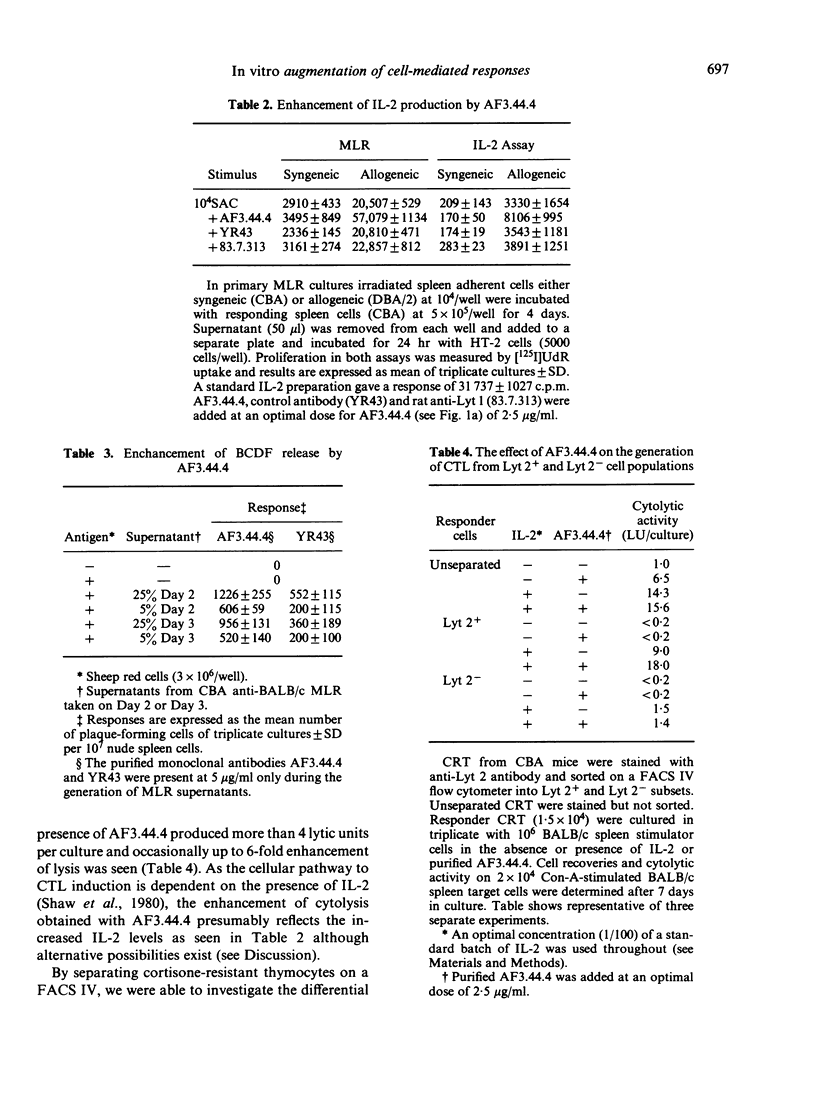
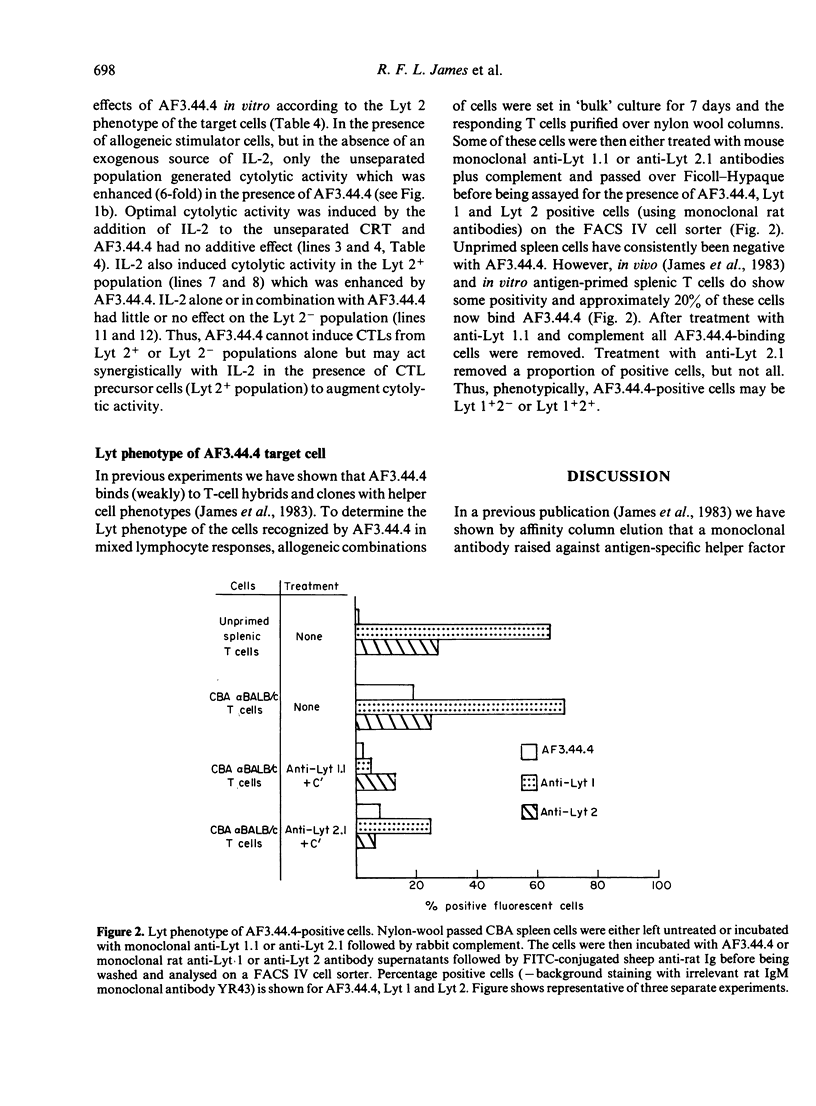
Previous studies have shown that monoclonal antibody AF3.44.4 has specificity for a constant region determinant on mouse antigen-specific helper factors and that it also binds to cultured T cells with functional helper cell characteristics. The antibody synergizes with antigen to enhance in vitro antibody responses; here we demonstrate that it will also enhance cell-mediated responses in vitro such as in the generation of proliferating cells in mixed lymphocyte responses and in the generation of specific killer cells in cytotoxic T lymphocyte cultures. The mechanism of AF3.44.4-generated enhancement was investigated. Increased levels of the lymphokines IL-2 and BCDF were detected in supernatants of AF3.44.4-treated cultures but the antibody itself could not replace interleukin-2 (IL-2), and would not stimulate primed cells in the absence of antigen. This type of monoclonal antibody which augments immunological responses in an antigen-dependent fashion may provide a new class of immunostimulant and a new approach to augmenting the responses of weak immunogens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Czitrom A. A., Katz D. R., Sunshine G. H. Alloreactive cytotoxic T lymphocyte responses to H-2 products on purified accessory cells. Immunology. 1982 Mar;45(3):553–560. [PMC free article] [PubMed] [Google Scholar]
- Czitrom A. A., Sunshine G. H., Reme T., Ceredig R., Glasebrook A. L., Kelso A., MacDonald H. R. Stimulator cell requirements for allospecific T cell subsets: specialized accessory cells are required to activate helper but not cytolytic T lymphocyte precursors. J Immunol. 1983 Feb;130(2):546–550. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Glasebrook A. L., Kelso A., MacDonald H. R. Cytolytic T lymphocyte clones that proliferate autonomously to specific alloantigenic stimulation. II. Relationship of the Lyt-2 molecular complex to cytolytic activity, proliferation, and lymphokine secretion. J Immunol. 1983 Apr;130(4):1545–1551. [PubMed] [Google Scholar]
- Gunter K. C., Malek T. R., Shevach E. M. T cell-activating properties of an anti-Thy-1 monoclonal antibody. Possible analogy to OKT3/Leu-4. J Exp Med. 1984 Mar 1;159(3):716–730. doi: 10.1084/jem.159.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt C., Röllinghoff M., Pfizenmaier K., Mosmann H., Wagner H. Lyt-23+ cyclophosphamide-sensitive T cells regulate the activity of an interleukin 2 inhibitor in vivo. J Exp Med. 1981 Aug 1;154(2):262–274. doi: 10.1084/jem.154.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins K., Kubo R., White J., Pigeon M., Kappler J., Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J Exp Med. 1983 Apr 1;157(4):1149–1169. doi: 10.1084/jem.157.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R. F., Kontiainen S., Maudsley D. J., Culbert E. J., Feldmann M. A monoclonal antibody against antigen-specific helper factor augments T-cell help. Nature. 1983 Jan 13;301(5896):160–163. doi: 10.1038/301160a0. [DOI] [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontiainen S., Feldmann M. Suppressor cell induction in vitro. I. Kinetics of induction of antigen-specific suppressor cells. Eur J Immunol. 1976 Apr;6(4):296–301. doi: 10.1002/eji.1830060412. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Larsson E. L. Functional heterogeneity of helper T cells: two distinct helper T cells are required for the production of T cell growth factor. J Immunol. 1982 Feb;128(2):742–745. [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Schwartz R. H. The use of antisera and monoclonal antibodies to identify the antigen-specific T cell receptor from pigeon cytochrome c-specific T cell hybrids. Immunol Rev. 1983;76:59–78. doi: 10.1111/j.1600-065x.1983.tb01097.x. [DOI] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. A third signal in B cell activation given by TRF. Transplant Rev. 1975;23:176–188. doi: 10.1111/j.1600-065x.1975.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Shaw J., Caplan B., Paetkau V., Pilarski L. M., Delovitch T. L., McKenzie I. F. Cellular origins of co-stimulator (IL2) and its activity in cytotoxic T lymphocyte responses. J Immunol. 1980 May;124(5):2231–2239. [PubMed] [Google Scholar]
- Sunshine G. H., Katz D. R., Czitrom A. A. Heterogeneity of stimulator cells in the murine mixed leukocyte response. Eur J Immunol. 1982 Jan;12(1):9–15. doi: 10.1002/eji.1830120105. [DOI] [PubMed] [Google Scholar]
- Tada T., Okumura K. The role of antigen-specific T cell factors in the immune response. Adv Immunol. 1979;28:1–87. doi: 10.1016/s0065-2776(08)60799-3. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Widmer M. B., Bach F. H. Antigen-driven helper cell-independent cloned cytolytic T lymphocytes. Nature. 1981 Dec 24;294(5843):750–752. doi: 10.1038/294750a0. [DOI] [PubMed] [Google Scholar]


