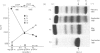Abstract
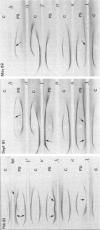
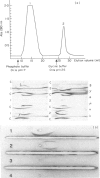
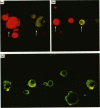
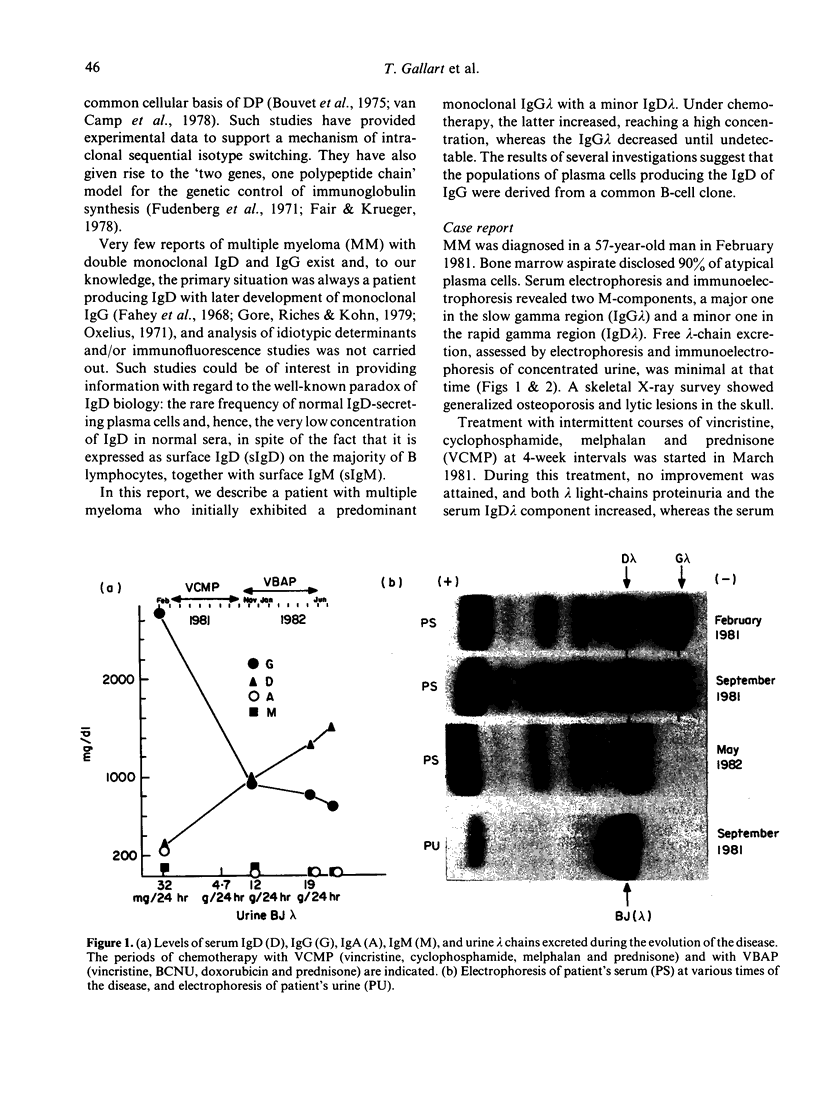
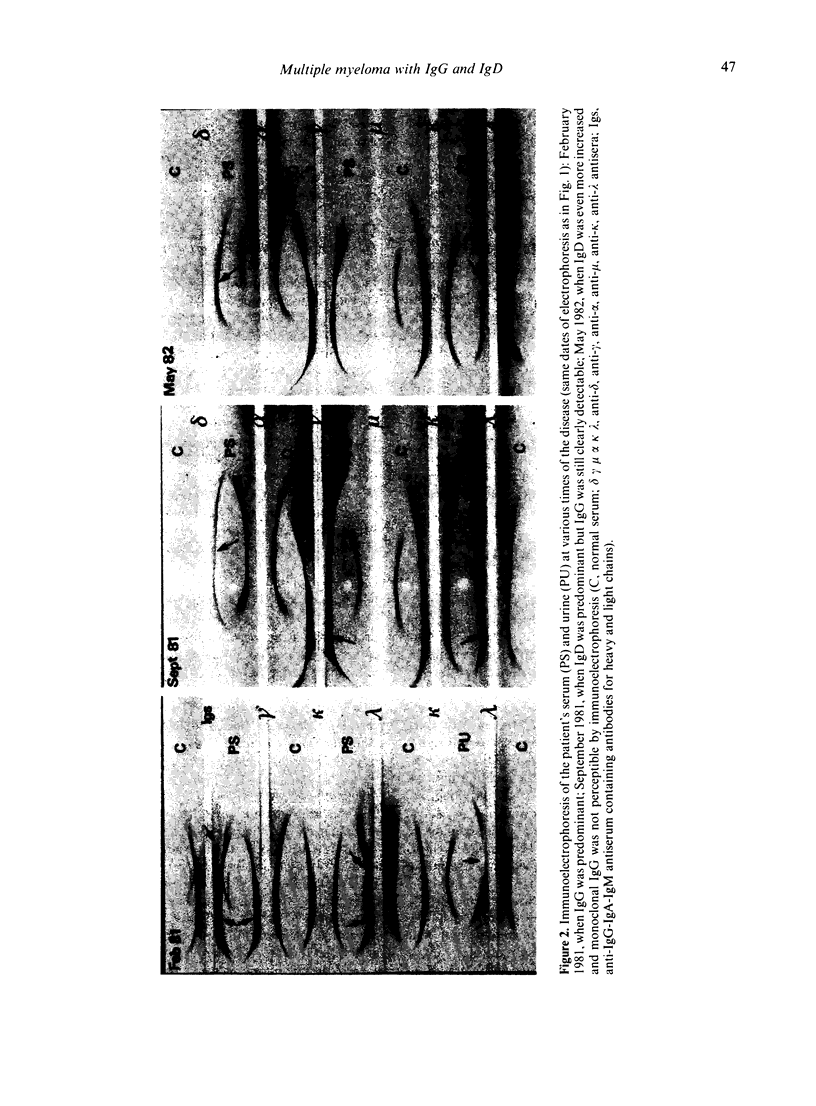
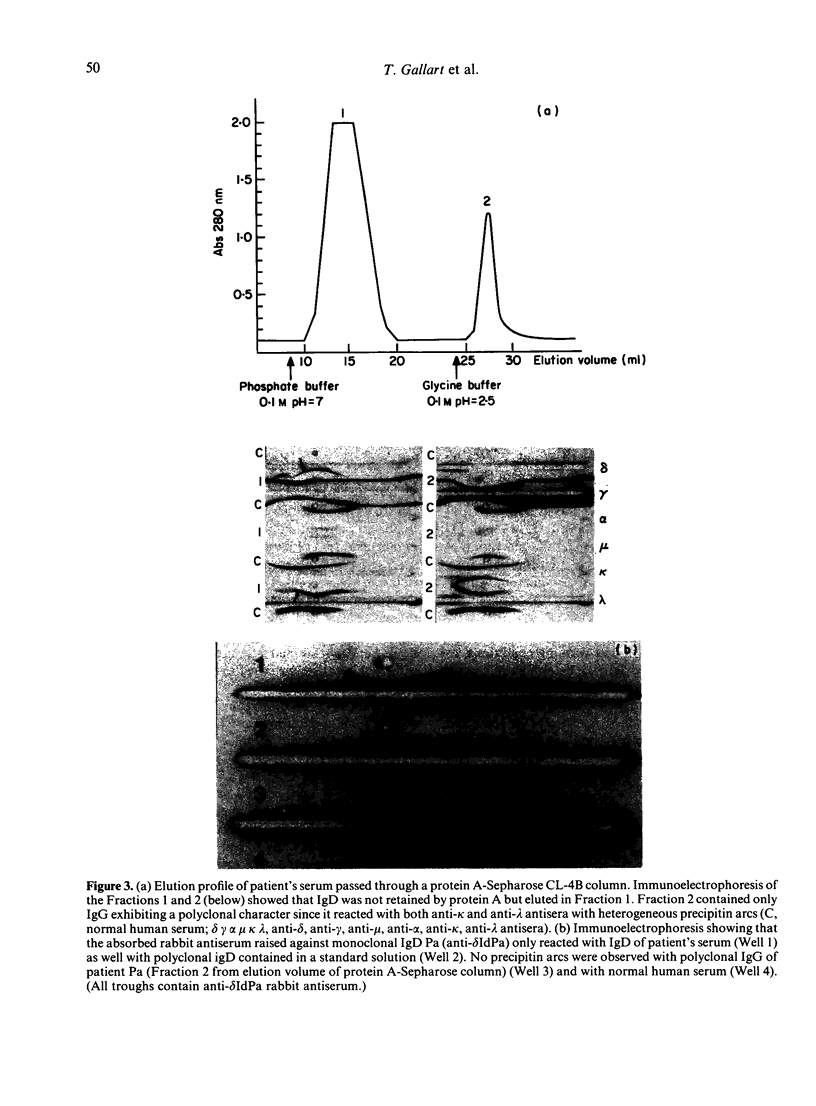
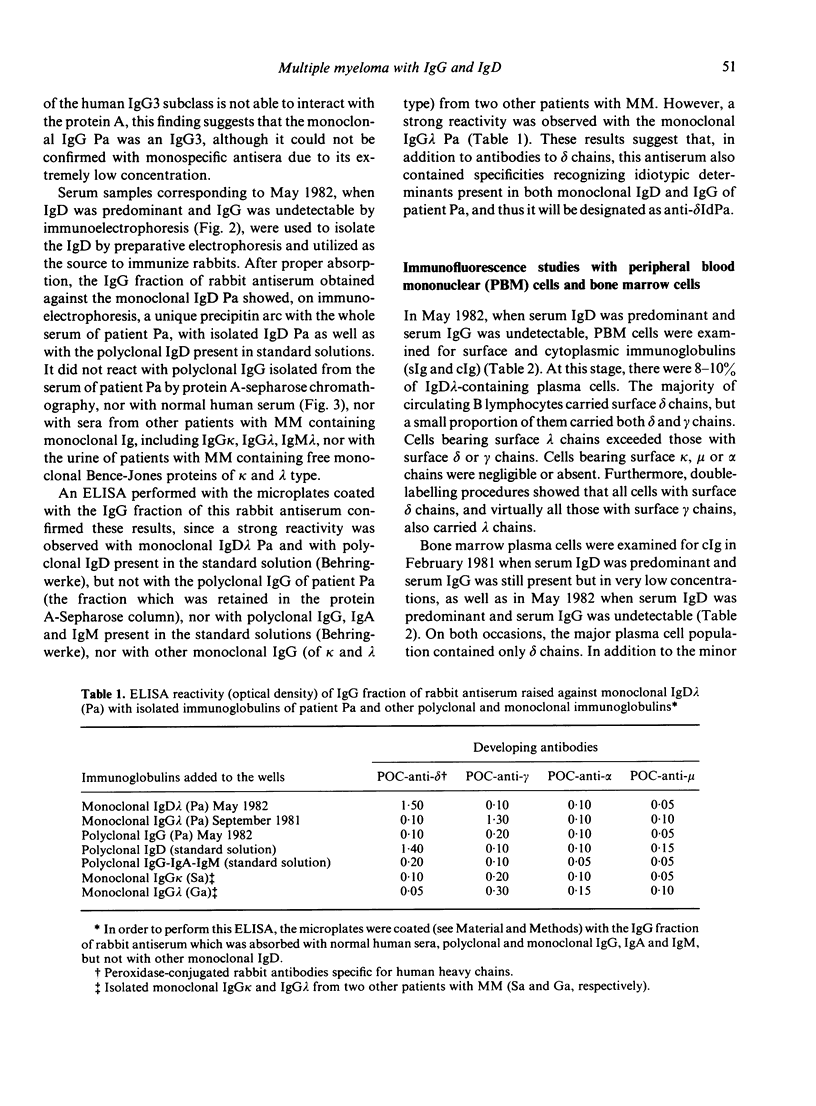
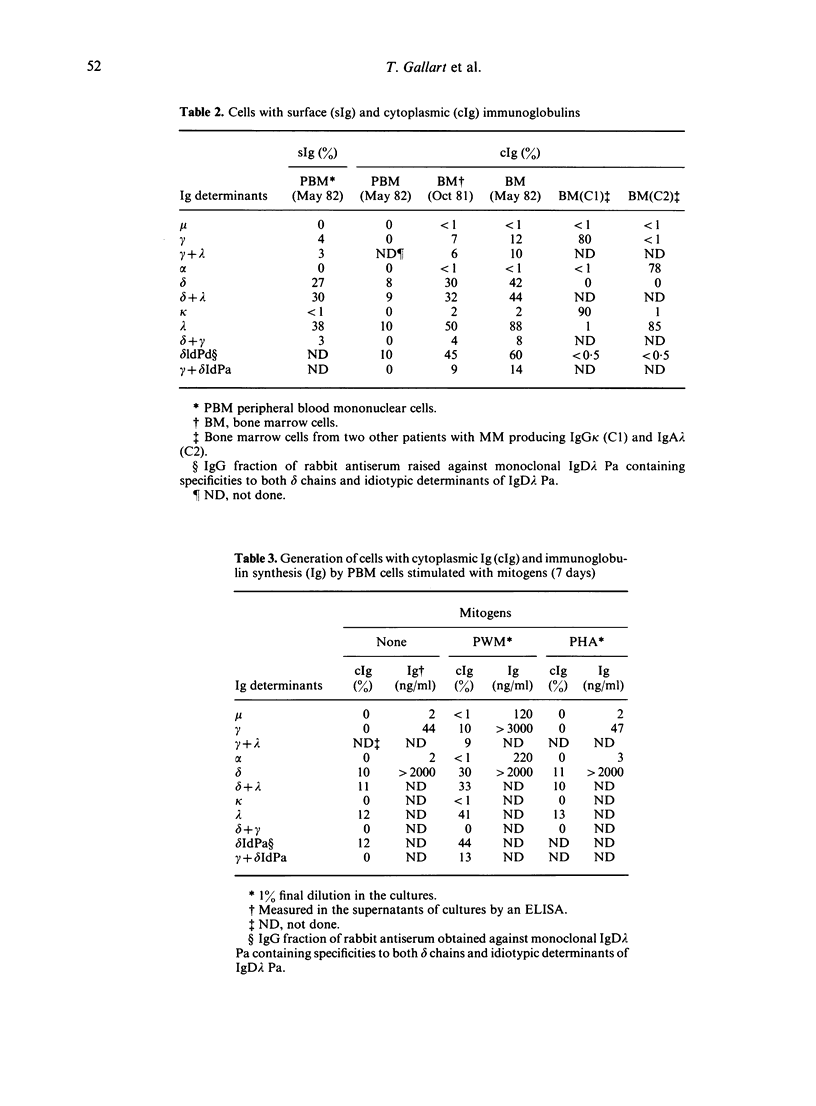
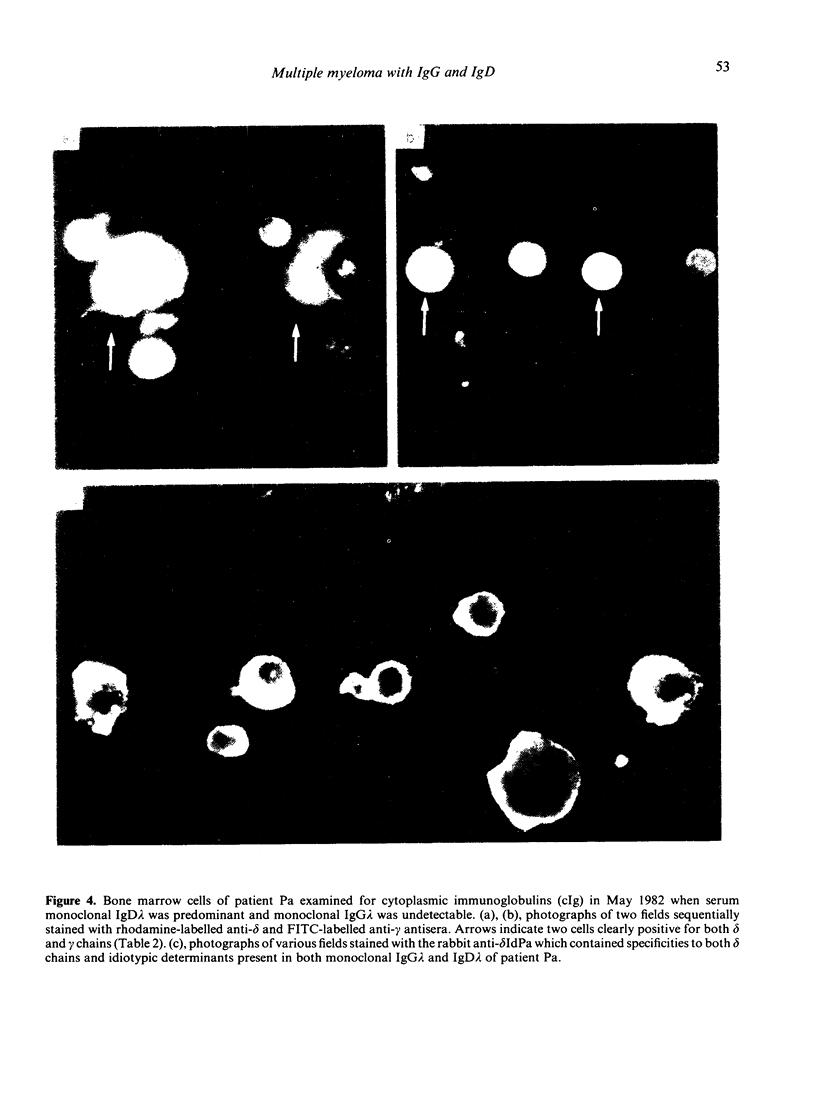
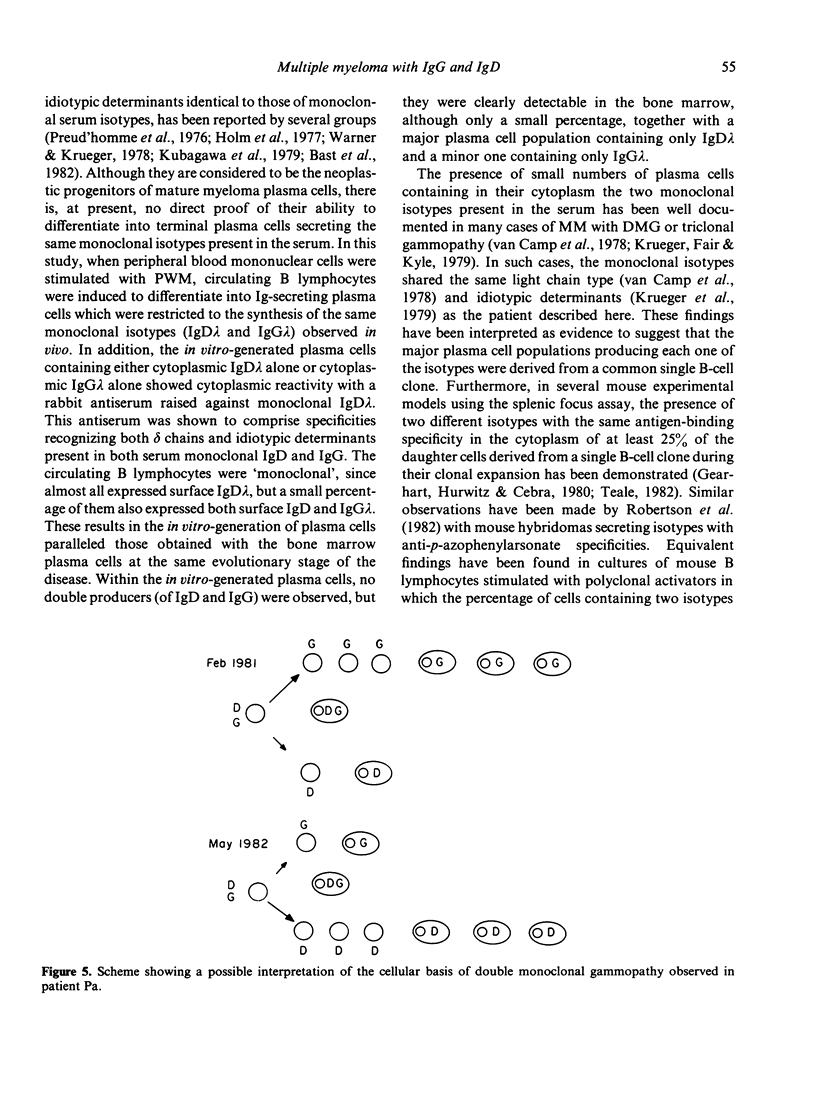
A patient with multiple myeloma (MM), who initially presented with a predominant IgG lambda and a minor IgD lambda paraprotein pattern, is described. After chemotherapy, levels of the IgD lambda protein increased and the IgG lambda levels decreased. The following results were obtained when serum IgD was predominant. In the bone marrow, there were three plasma cell populations: a major one containing only delta chains, a minor one containing only gamma chains, and another minor one containing both delta and gamma chains. All these plasma cell populations contained lambda chains. Stimulation of circulating mononuclear cells with pokeweed mitogen (PWM) achieved differentiation of circulating B lymphocytes into plasma cells: 30% with only cytoplasmic delta lambda chains and 10% with only cytoplasmic gamma lambda chains. These IgG-containing plasma cells showed cytoplasmic reactivity with rabbit antiserum raised against monoclonal IgD which was shown to contain specificities recognizing both delta chains and idiotypic determinants present in both serum IgD lambda and IgG lambda. Circulating B lymphocytes were 'monoclonal': almost all expressed surface delta lambda chains, and a small proportion of them expressed both delta gamma and lambda chains. High levels of IgD were detected in the supernatants of all cultures, but high concentrations of IgG were only detected in those from PWM-stimulated cultures with very low levels of IgM and IgA. These findings suggest that plasma cells producing either IgD or IgG were derived from a common B-cell clone. Double paraproteinaemia exhibiting a shift in immunoglobulin production from IgG to IgD has not been previously described.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bast E. J., van Camp B., Reynaert P., Wiringa G., Ballieux R. E. Idiotypic peripheral blood lymphocytes in monoclonal gammopathy. Clin Exp Immunol. 1982 Mar;47(3):677–682. [PMC free article] [PubMed] [Google Scholar]
- Bouvet J. P., Feingold J., Oriol R., Liacopoulos P. Statistical study on double paraproteinemias. Evidence for a common cellular origin of both myeloma globulins. Biomedicine. 1975 Nov;22(6):517–523. [PubMed] [Google Scholar]
- Cooper M. D., Kuritani T., Chen C., Lehmeyer J. E., Gathings W. E. Expression of IgD as a function of B-cell differentiation. Ann N Y Acad Sci. 1982;399:146–156. doi: 10.1111/j.1749-6632.1982.tb25670.x. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fahey J. L., Carbone P. P., Rowe D. S., Bachmann R. Plasma cell myeloma with D-myeloma protein (IgD myeloma). Am J Med. 1968 Sep;45(3):373–380. doi: 10.1016/0002-9343(68)90071-5. [DOI] [PubMed] [Google Scholar]
- Fair D. S., Krueger R. G. Analysis of biclonal immunoglobulins and their contributions to understanding the developmental aspects of the antibody response. Contemp Top Mol Immunol. 1978;7:51–93. doi: 10.1007/978-1-4757-0779-3_2. [DOI] [PubMed] [Google Scholar]
- Fair D. S., Schaffer S., Krueger R. G. Development of monoclonal IgA and an apparent IgG in a patient with macroglobulinemia: sharing of individually specific antigenic determinants among IgM, IgA, and IgG. J Immunol. 1976 Sep;117(3):944–949. [PubMed] [Google Scholar]
- Fudenberg H. H., Wang A. C., Pink J. R., Levin A. S. Studies of an unusual biclonal gammopathy: implications with regard to genetic control of normal immunoglobulin synthesis. Ann N Y Acad Sci. 1971 Dec 31;190:501–506. doi: 10.1111/j.1749-6632.1971.tb13559.x. [DOI] [PubMed] [Google Scholar]
- Gathings W. E., Lawton A. R., Cooper M. D. Immunofluorescent studies of the development of pre-B cells, B lymphocytes and immunoglobulin isotype diversity in humans. Eur J Immunol. 1977 Nov;7(11):804–810. doi: 10.1002/eji.1830071112. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Hurwitz J. L., Cebra J. J. Successive switching of antibody isotypes expressed within the lines of a B-cell clone. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5424–5428. doi: 10.1073/pnas.77.9.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore M. E., Riches P. G., Kohn J. Identification of the paraproteins and clinical significance of more than one paraprotein and clinical significance of more than one paraprotein in serum of 56 patients. J Clin Pathol. 1979 Apr;32(4):313–317. doi: 10.1136/jcp.32.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia J., Rubies-Prat J., Gallart M. T., Moragas A., Martinez-Vazquez J. M., Bacardi R., Vilaseca J. The evolution of alpha heavy chain disease. Am J Med. 1976 Apr;60(4):596–602. doi: 10.1016/0002-9343(76)90729-4. [DOI] [PubMed] [Google Scholar]
- Holm G., Mellstedt H., Pettersson D., Biberfeld R. Idiotypic immunoglobulin structures on blood lymphocytes in human plasma cell myeloma. Immunol Rev. 1977;34:139–164. doi: 10.1111/j.1600-065x.1977.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Honjo T., Nakai S., Nishida Y., Kataoka T., Yamawaki-Kataoka Y., Takahashi N., Obata M., Shimizu A., Yaoita Y., Nikaido T. Rearrangements of immunoglobulin genes during differentiation and evolution. Immunol Rev. 1981;59:33–67. doi: 10.1111/j.1600-065x.1981.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Hopper J. E., Haren J. M., Kmiecik T. E. Evidence for shared idiotypy expressed by the IgM, IgG, and IgA serum proteins of a patient with a complex multiple paraprotein disorder. J Immunol. 1979 May;122(5):2000–2006. [PubMed] [Google Scholar]
- Krueger R. G., Fair D. S., Kyle R. A. Monoclonal IgM, IgA and IgG in the serum of a single individual: immunofluorescence identification of cells producing the immunoglobulins. Eur J Immunol. 1979 Aug;9(8):602–606. doi: 10.1002/eji.1830090806. [DOI] [PubMed] [Google Scholar]
- Kubagawa H., Vogler L. B., Capra J. D., Conrad M. E., Lawton A. R., Cooper M. D. Studies on the clonal origin of multiple myeloma. Use of individually specific (idiotype) antibodies to trace the oncogenic event to its earliest point of expression in B-cell differentiation. J Exp Med. 1979 Oct 1;150(4):792–807. doi: 10.1084/jem.150.4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle R. A., Robinson R. A., Katzmann J. A. The clinical aspects of biclonal gammopathies. Review of 57 cases. Am J Med. 1981 Dec;71(6):999–1008. doi: 10.1016/0002-9343(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Nieto A., Gaya A., Jansa M., Moreno C., Vives J. Direct measurement of antibody affinity distribution by hapten-inhibition enzyme immunoassay. Mol Immunol. 1984 Jun;21(6):537–543. doi: 10.1016/0161-5890(84)90070-1. [DOI] [PubMed] [Google Scholar]
- Obata M., Kataoka T., Nakai S., Yamagishi H., Takahashi N., Yamawaki-Kataoka Y., Nikaido T., Shimizu A., Honjo T. Structure of a rearranged gamma 1 chain gene and its implication to immunoglobulin class-switch mechanism. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2437–2441. doi: 10.1073/pnas.78.4.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxelius V. A. Alternating appearance of IgD and IgG myeloma protein during treatment. Scand J Haematol. 1971;8(6):439–445. doi: 10.1111/j.1600-0609.1971.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Cooper M. D. A model for the differentiation of B lymphocytes with implications for the biological role of IgD. Immunol Rev. 1977;37:105–126. doi: 10.1111/j.1600-065x.1977.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Preud'Homme J. L., Brouet J. C., Seligmann M. Membrane-bound IgD on human lymphoid cells, with special reference to immunodeficiency and immunoproliferative diseases. Immunol Rev. 1977;37:127–151. doi: 10.1111/j.1600-065x.1977.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Preud'Homme J. L., Hurez D., Danon F., Brouet J. C., Seligmann M. Intracytoplasmic and surface-bound immunoglobulins in "nonsecretory" and Bence-Jones myeloma. Clin Exp Immunol. 1976 Sep;25(3):428–436. [PMC free article] [PubMed] [Google Scholar]
- Robertson S. M., Clayton L., Dev V. G., Wall R., Capra J. D., Kettman J. R. Antiarsonate antibody response: a model for studying antibody diversity. Fed Proc. 1982 Jul;41(9):2502–2506. [PubMed] [Google Scholar]
- Severinson E., Bergstedt-Lindqvist S., van der Loo W., Fernandez C. Characterization of the IgG response induced by polyclonal B cell activators. Immunol Rev. 1982;67:73–85. doi: 10.1111/j.1600-065x.1982.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Teale J. M. The potential of B lymphocytes for isotype expression. Fed Proc. 1982 Jul;41(9):2497–2501. [PubMed] [Google Scholar]
- Vitetta E. S. Immunoglobulin receptors reevaluated. Ann N Y Acad Sci. 1982;399:255–264. doi: 10.1111/j.1749-6632.1982.tb25678.x. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. IgD and B cell differentiation. Immunol Rev. 1977;37:50–88. doi: 10.1111/j.1600-065x.1977.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Warner T. F., Krueger R. G. Circulating lymphocytes and the spread of myeloma. Review of the evidence. Lancet. 1978 Jun 3;1(8075):1174–1176. doi: 10.1016/s0140-6736(78)90966-2. [DOI] [PubMed] [Google Scholar]
- Wolfenstein-Todel C., Franklin E. C., Rudders R. A. Similarities of the light chains and the variable regions of the heavy chains of the IgG2 lambda and IgA1 lambda myeloma proteins from a single patient. J Immunol. 1974 Mar;112(3):871–876. [PubMed] [Google Scholar]
- Yagi Y., Pressman D. Monoclonal IgA and IgM in the serum of a single patient (SC). I. Sharing of individually specific determinants between IgA and IgM. J Immunol. 1973 Feb;110(2):335–344. [PubMed] [Google Scholar]
- van der Loo W., Gronowicz E. S., Strober S., Herzenberg L. A. Cell differentiation in the presence of cytochalasin B: studies on the "switch" to IgG secretion after polyclonal B cell activation. J Immunol. 1979 Apr;122(4):1203–1208. [PubMed] [Google Scholar]