Abstract
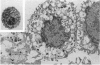
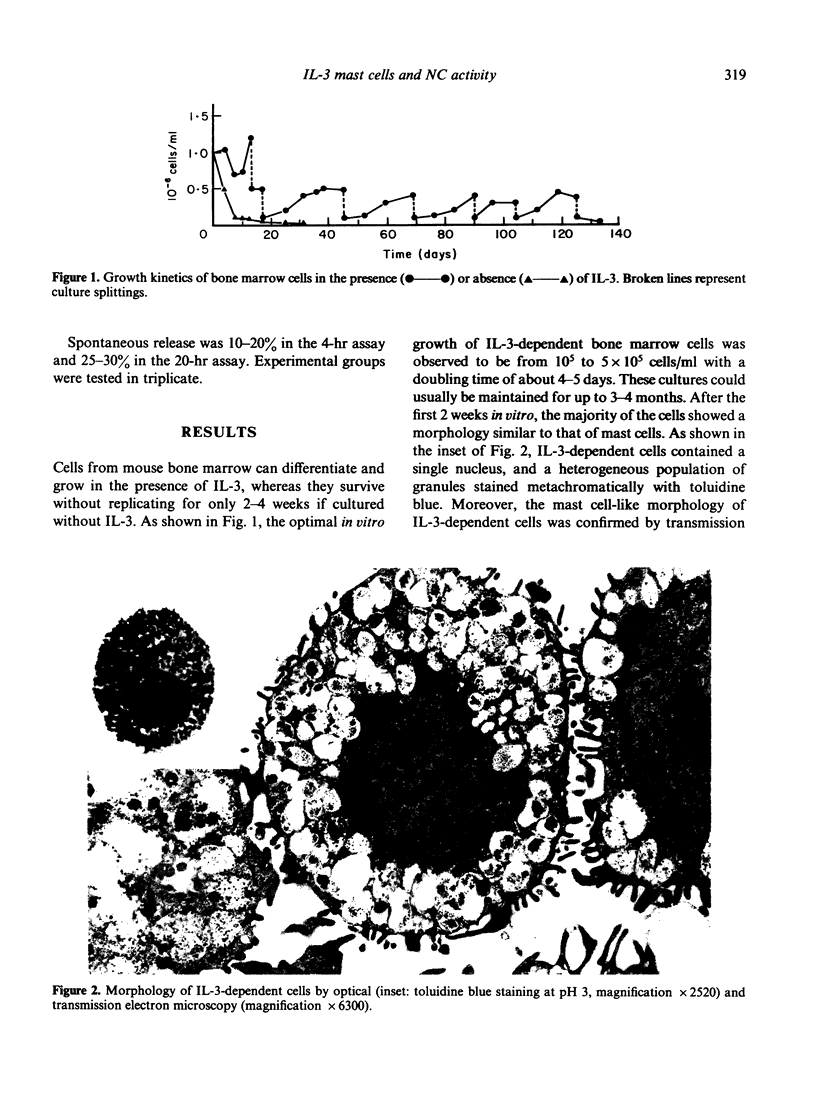
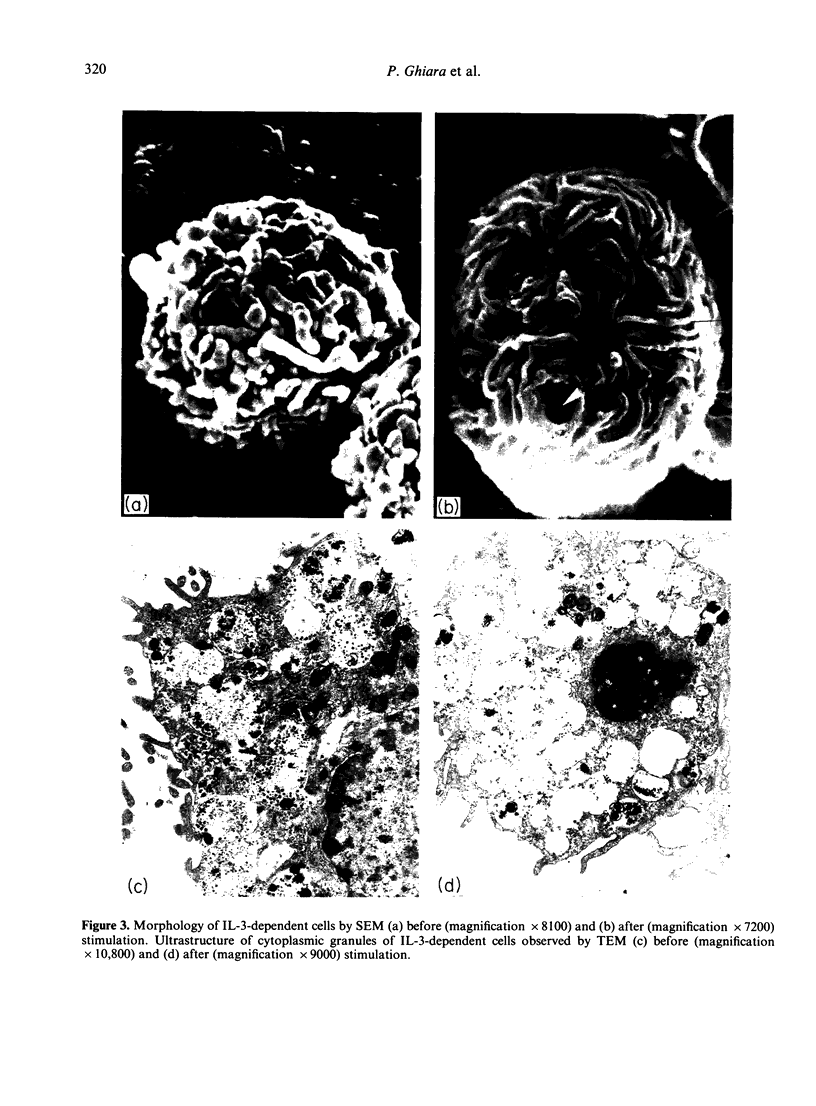
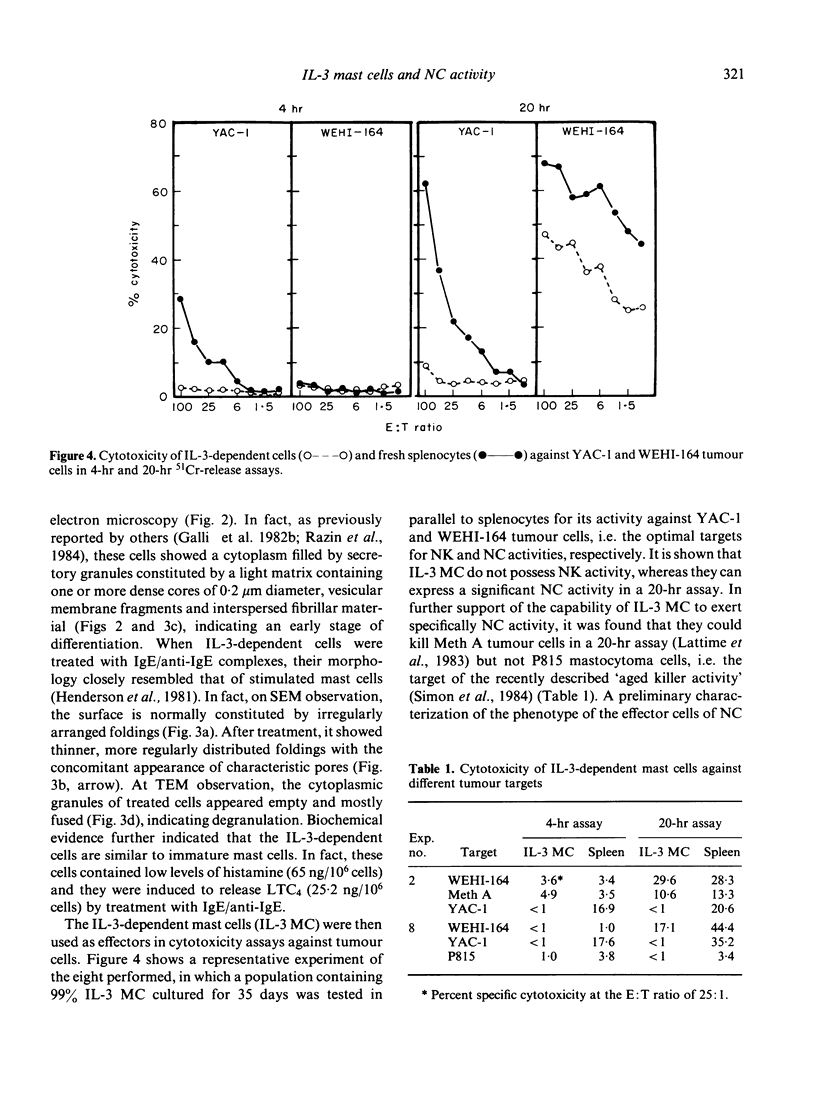
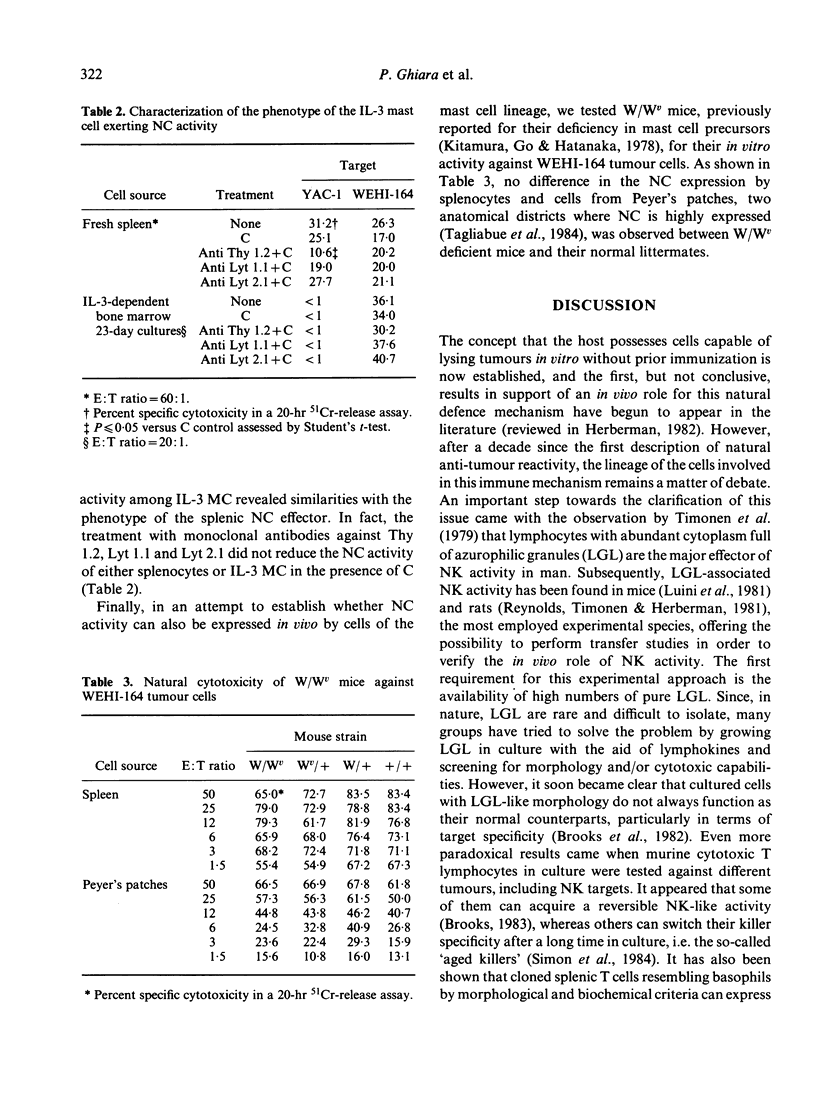
Bone marrow cells from mice were cultured in vitro in the presence of interleukin-3 (IL-3). After 2 weeks, the majority of the cells differentiated towards mast cells as judged by morphological and biochemical criteria. When populations of 99% IL-3-dependent mast cells (IL-3 MC) were tested for their anti-tumour activity in vitro, it was found that they can express natural cytotoxicity (NC) but not other natural reactivities. Moreover, the effector cells were not positive for Thy 1, Lyt 1 and Lyt 2 markers. The capability of mast cells to express NC activity seems to be related to their in vitro differentiation, since mast cell deficient W/Wv mice had normal NC activity. Thus, IL-3 MC must be added to the variety of cells capable of expressing natural anti-tumour reactivities in vitro.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anton A. H., Sayre D. F. A modified fluorometric procedure for tissue histamine and its distribution in various animals. J Pharmacol Exp Ther. 1969 Apr;166(2):285–290. [PubMed] [Google Scholar]
- Brooks C. G., Kuribayashi K., Sale G. E., Henney C. S. Characterization of five cloned murine cell lines showing high cytolytic activity against YAC-1 cells. J Immunol. 1982 May;128(5):2326–2335. [PubMed] [Google Scholar]
- Brooks C. G. Reversible induction of natural killer cell activity in cloned murine cytotoxic T lymphocytes. Nature. 1983 Sep 8;305(5930):155–158. doi: 10.1038/305155a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu J. Y., Lanza E., Pastore S., Hapel A. J. Selective growth of natural cytotoxic but not natural killer effector cells in interleukin-3. Nature. 1983 Dec 22;306(5945):788–791. doi: 10.1038/306788a0. [DOI] [PubMed] [Google Scholar]
- Farram E., Nelson D. S. Mouse mast cells as anti-tumor effector cells. Cell Immunol. 1980 Oct;55(2):294–301. doi: 10.1016/0008-8749(80)90162-8. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Ishizaka T., Nabel G., Der Simonian H., Cantor H., Dvorak H. F. A cloned cell with NK function resembles basophils by ultrastructure and expresses IgE receptors. Nature. 1982 Jul 15;298(5871):288–290. doi: 10.1038/298288a0. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Marcum J. A., Ishizaka T., Nabel G., Der Simonian H., Pyne K., Goldin J. M., Rosenberg R. D., Cantor H. Mast cell clones: a model for the analysis of cellular maturation. J Cell Biol. 1982 Nov;95(2 Pt 1):435–444. doi: 10.1083/jcb.95.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson W. R., Chi E. Y., Jong E. C., Klebanoff S. J. Mast cell-mediated tumor-cell cytotoxicity. Role of the peroxidase system. J Exp Med. 1981 Mar 1;153(3):520–533. doi: 10.1084/jem.153.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975 Aug 15;16(2):230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Oroszlan S., Henderson L. E., Copeland T. D., Fitch F., Prystowsky M. B., Goldwasser E., Schrader J. W., Palaszynski E. Biologic properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol. 1983 Jul;131(1):282–287. [PubMed] [Google Scholar]
- Ihle J. N., Pepersack L., Rebar L. Regulation of T cell differentiation: in vitro induction of 20 alpha-hydroxysteroid dehydrogenase in splenic lymphocytes from athymic mice by a unique lymphokine. J Immunol. 1981 Jun;126(6):2184–2189. [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978 Aug;52(2):447–452. [PubMed] [Google Scholar]
- Lattime E. C., Pecoraro G. A., Stutman O. The activity of natural cytotoxic cells is augmented by interleukin 2 and interleukin 3. J Exp Med. 1983 Mar 1;157(3):1070–1075. doi: 10.1084/jem.157.3.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini W., Boraschi D., Alberti S., Aleotti A., Tagliabue A. Morphological characterization of a cell population responsible for natural killer activity. Immunology. 1981 Aug;43(4):663–668. [PMC free article] [PubMed] [Google Scholar]
- Lust J. A., Kumar V., Burton R. C., Bartlett S. P., Bennett M. Heterogeneity of natural killer cells in the mouse. J Exp Med. 1981 Aug 1;154(2):306–317. doi: 10.1084/jem.154.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato N., Reid L., Bloom B. R. On the heterogeneity of murine natural killer cells. J Exp Med. 1981 Sep 1;154(3):750–762. doi: 10.1084/jem.154.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Konigsberg P. J. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J Exp Med. 1984 Sep 1;160(3):695–710. doi: 10.1084/jem.160.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin E., Ihle J. N., Seldin D., Mencia-Huerta J. M., Katz H. R., LeBlanc P. A., Hein A., Caulfield J. P., Austen K. F., Stevens R. L. Interleukin 3: A differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J Immunol. 1984 Mar;132(3):1479–1486. [PubMed] [Google Scholar]
- Reynolds C. W., Timonen T., Herberman R. B. Natural killer (NK) cell activity in the rat. I. Isolation and characterization of the effector cells. J Immunol. 1981 Jul;127(1):282–287. [PubMed] [Google Scholar]
- Simon M. M., Weltzien H. U., Bühring H. J., Eichmann K. Aged murine killer T-cell clones acquire specific cytotoxicity for P815 mastocytoma cells. Nature. 1984 Mar 22;308(5957):367–370. doi: 10.1038/308367a0. [DOI] [PubMed] [Google Scholar]
- Tagliabue A., Befus A. D., Clark D. A., Bienenstock J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J Exp Med. 1982 Jun 1;155(6):1785–1796. doi: 10.1084/jem.155.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabue A., Mantovani A., Kilgallen M., Herberman R. B., McCoy J. L. Natural cytotoxicity of mouse monocytes and macrophages. J Immunol. 1979 Jun;122(6):2363–2370. [PubMed] [Google Scholar]
- Tagliabue A., Villa L., Scapigliati G., Boraschi D. Peyer's patch lymphocytes express natural cytotoxicity but not natural killer activity. Nat Immun Cell Growth Regul. 1983;3(2):95–101. [PubMed] [Google Scholar]
- Timonen T., Saksela E., Ranki A., Häyry P. Fractionation, morphological and functional characterization of effector cells responsible for human natural killer activity against cell-line targets. Cell Immunol. 1979 Nov;48(1):133–148. doi: 10.1016/0008-8749(79)90106-0. [DOI] [PubMed] [Google Scholar]