Abstract
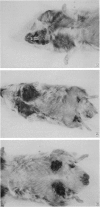
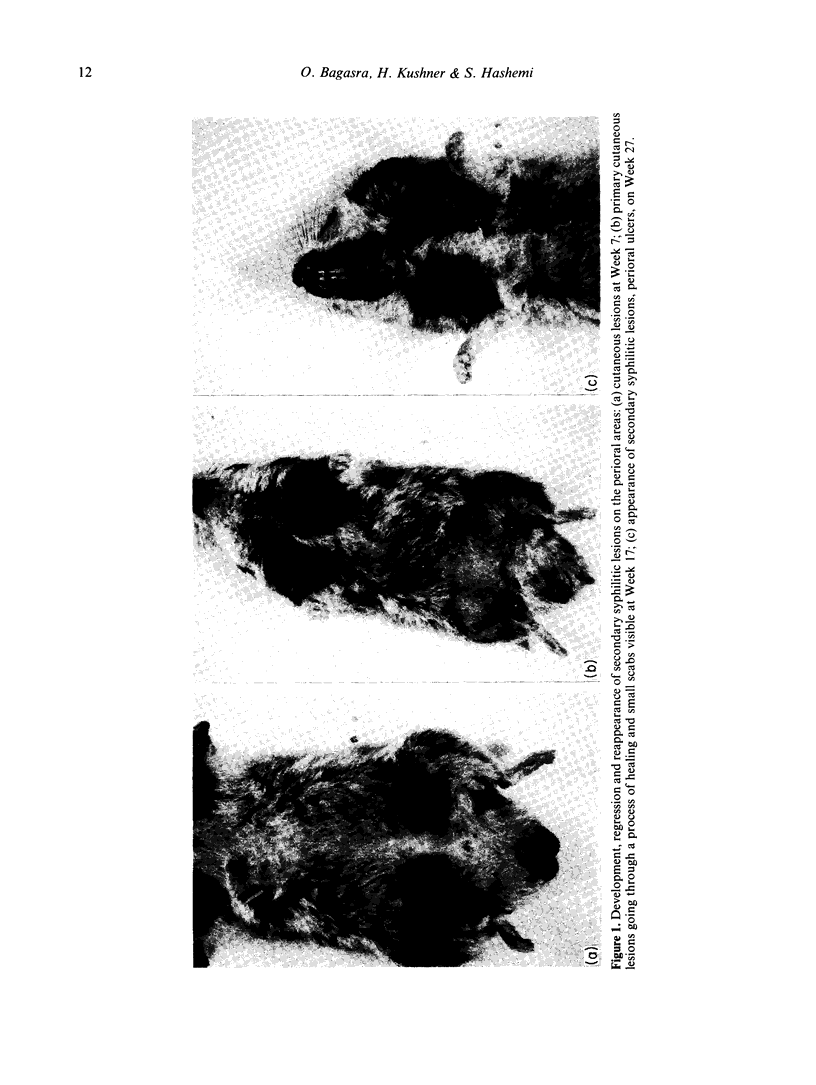
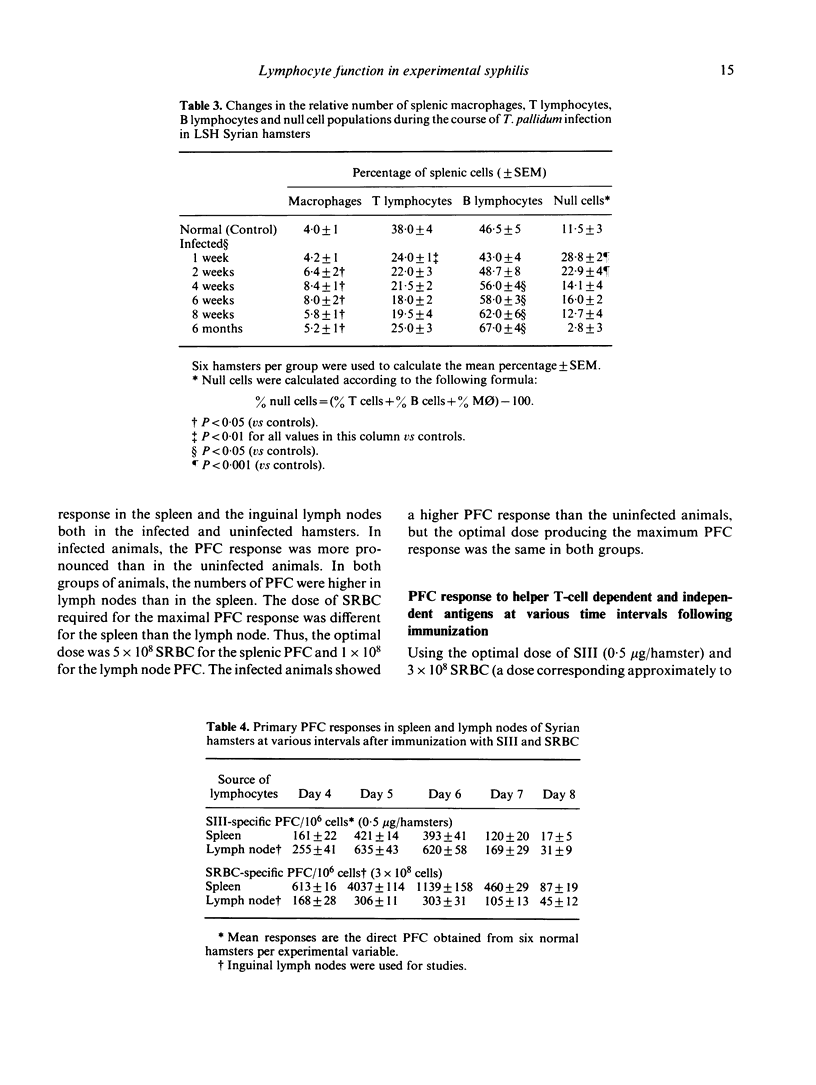
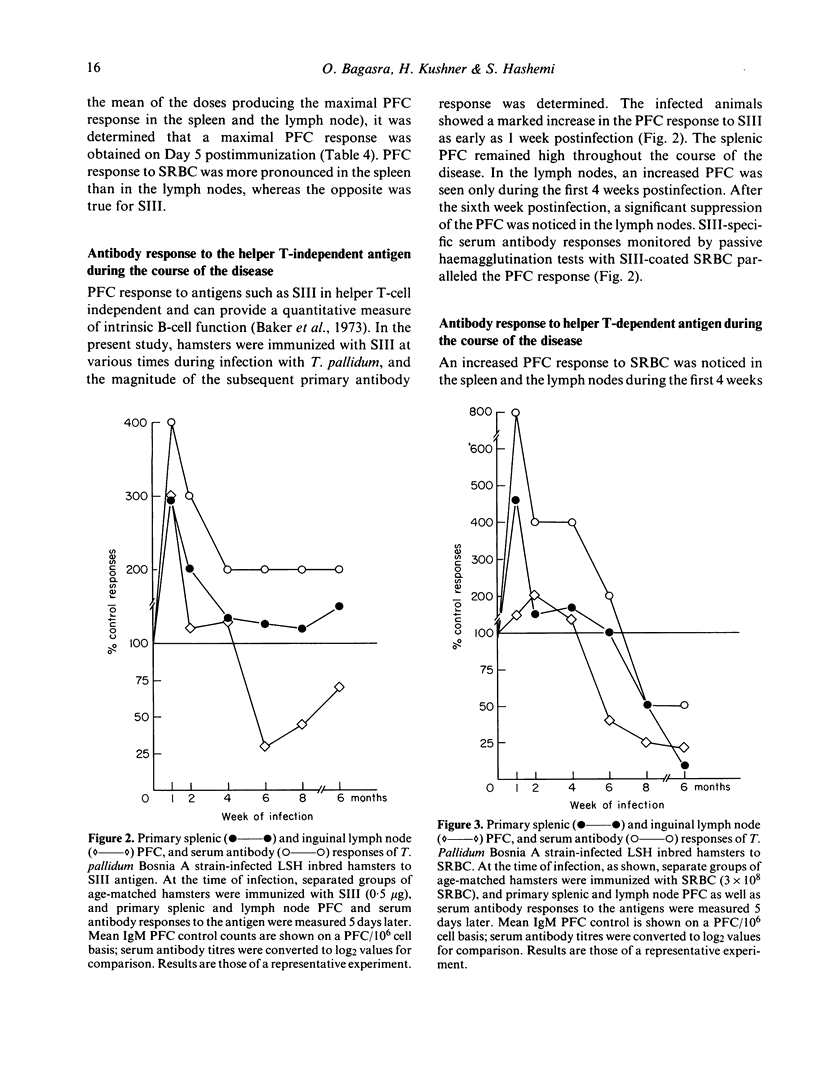
We have studied the changes in the lymph nodes, spleen and thymus that occur in inbred LSH Syrian hamsters infected with Treponema pallidum Bosnia A, the causative agent of endemic syphilis, as well as the B-cell responses of these infected animals to helper T-cell independent and dependent antigens. The lymph nodes increased significantly in weight up to 6 weeks after infection, and contained viable treponemes. No significant changes in the spleen weight were observed, and no viable treponemes could be recovered from the spleen. However, the size of the thymus decreased steadily during the course of the disease. The relative number of Ig+ cells (B cells) increased in the spleen and regional lymph nodes, whereas the relative number of T cells decreased during the course of infection. In both the spleen and lymph nodes, the relative number of macrophages increased initially and decreased thereafter in the form of a bell-shaped curve showing a peak at 4-6 weeks of infection. The ability of splenic lymphocytes from infected hamsters to mount a primary PFC response to pneumococcal polysaccharide type III (SIII), a helper T-cell independent antigen, was elevated throughout the course of infection. However, the splenic PFC response to sheep erythrocytes (SRBC), a helper T-cell dependent antigen, was increased only during the first 4 weeks of infection and progressively decreased thereafter. The PFC responses of infected lymph node lymphocytes to both SIII and SRBC were increased during the first 4 weeks and decreased thereafter. These data suggested that atrophy of the thymus seen in syphilitic infection is accompanied by the complex losses of subsets of T cells and altered B-cell functions. An early loss of suppressor T cells in both the lymph nodes and spleen occurs concomitantly with a loss of T helper cells and heterologous (treponema-unrelated) B-cell functions in the lymph nodes. Helper T cells are lost from the spleen only in the later stages of infection, whereas splenic B-cell functions remain intact throughout the course of the disease. These findings were further tested by in vitro methods where splenic and lymph node lymphocytes from infected hamsters were examined for their ability to respond to Con A in terms of the induction of antigen non-specific suppressor T cells. The mixing of Con A stimulated splenic or lymph node lymphocytes from infected hamsters was unable to inhibit the primary antibody responses of SRBC as compared to the normal control.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagasra O., Damjanov I. Ability of macrophages to process and present Treponema pallidum Bosnia A strain antigens in experimental syphilis of syrian hamsters. Infect Immun. 1982 Apr;36(1):176–183. doi: 10.1128/iai.36.1.176-183.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Reed N. D., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. I. Nature of regulatory cells. J Exp Med. 1973 Jun 1;137(6):1431–1441. doi: 10.1084/jem.137.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. II. Mode of action of thymic-derived suppressor cells. J Immunol. 1974 Jan;112(1):404–409. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Musher D. M. Aberrant secondary antibody responses to sheep erythrocytes in rabbits with experimental syphilis. Infect Immun. 1979 Jul;25(1):133–138. doi: 10.1128/iai.25.1.133-138.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Musher D. M. Altered immune responsiveness associated with experimental syphilis in the rabbit: elevated IgM and depressed IgG responses to sheep erythrocytes. J Immunol. 1978 May;120(5):1691–1695. [PubMed] [Google Scholar]
- Baughn R. E., Musher D. M., Simmons C. B. Inability of spleen cells from chancre-immune rabbits to confer immunity to challenge with Treponema pallidum. Infect Immun. 1977 Sep;17(3):535–540. doi: 10.1128/iai.17.3.535-540.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Tung K. S., Musher D. M. Detection of circulating immune complexes in the sera of rabbits with experimental syphilis: possible role in immunoregulation. Infect Immun. 1980 Aug;29(2):575–582. doi: 10.1128/iai.29.2.575-582.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. I. The demonstration of resistance conferred by passive immunization. J Immunol. 1976 Jul;117(1):191–196. [PubMed] [Google Scholar]
- CSONKA G. W. 'Luotest' a preliminary evaluation in the diagnosis of late syphilis. Med Illus. 1950 Aug;4(8):389–391. [PubMed] [Google Scholar]
- Fiumara N. J., Lessell S. Manifestations of late congenital syphilis. An analysis of 271 patients. Arch Dermatol. 1970 Jul;102(1):78–83. [PubMed] [Google Scholar]
- Fulford K. W., Brostoff J. Leucocyte migration and cell-mediated immunity in syphilis. Br J Vener Dis. 1972 Dec;48(6):483–488. doi: 10.1136/sti.48.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine W. J., Stewart A. G., Scarth L. A clinical and immunological study of adrenocortical insufficiency (Addison's disease). Clin Exp Immunol. 1967 Jan;2(1):31–70. [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. C., Axelrad M. A. An improved assay for haemolytic plaque-forming cells. Immunology. 1971 Feb;20(2):253–257. [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Enhanced immune responsiveness to a thymus-independent antigen early after adult thymectomy: evidence for short-lived inhibitory thymus-derived cells. Eur J Immunol. 1972 Apr;2(2):114–118. doi: 10.1002/eji.1830020204. [DOI] [PubMed] [Google Scholar]
- Kong A. S., Morse S. I. The effect of Bordetella pertussis on the antibody response in mice to type III pneumococcal polysaccharide. J Immunol. 1976 Apr;116(4):989–993. [PubMed] [Google Scholar]
- Levene G. M., Wright D. J., Turk J. L. Cell-mediated immunity and lymphocyte transformation in syphilis. Proc R Soc Med. 1971 Apr;64(4):426–428. [PMC free article] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Effect of cortisone administration on host-parasite relationships in early experimental syphilis. J Immunol. 1981 Oct;127(4):1361–1368. [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J Immunol. 1980 Jan;124(1):454–460. [PubMed] [Google Scholar]
- Maret S. M., Baseman J. B., Folds J. D. Cell-mediated immunity in Treponema pallidum infected rabbits: in vitro response of splenic and lymph node lymphocytes to mitogens and specific antigens. Clin Exp Immunol. 1980 Jan;39(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- Metzger M., Podwińska J., Smogór W. Development of immunological responsiveness and resistance to infection with Treponema pallidum in rabbits given immune lymphocyte preparations. Arch Immunol Ther Exp (Warsz) 1980;28(2):329–336. [PubMed] [Google Scholar]
- Miller J. N. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by -irradiation. J Immunol. 1973 May;110(5):1206–1215. [PubMed] [Google Scholar]
- Noguchi H. A CUTANEOUS REACTION IN SYPHILIS. J Exp Med. 1911 Dec 1;14(6):557–568. doi: 10.1084/jem.14.6.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Baseman J. B., Folds J. D. Selective response of lymphocytes from Treponema pallidum-infected rabbits to mitogens and Treponema reiteri. Infect Immun. 1977 Feb;15(2):417–422. doi: 10.1128/iai.15.2.417-422.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Depression of lymphocyte response to concanavalin A in rabbits infected with Treponema pallidum (Nichols strain). Infect Immun. 1976 Jul;14(1):320–322. doi: 10.1128/iai.14.1.320-322.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perine P. L., Weiser R. S., Klebanoff S. J. Immunity to syphilis. I. Passive transfer in rabbits with hyperimmune serum. Infect Immun. 1973 Nov;8(5):787–790. doi: 10.1128/iai.8.5.787-790.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Roelants G. E., Pearson T. W., Tyrer H. W., Mayor-Withey K. S., Lundin L. B. Immune depression in trypanosome-infected mice. II. Characterization of the spleen cell types involved. Eur J Immunol. 1979 Mar;9(3):195–199. doi: 10.1002/eji.1830090305. [DOI] [PubMed] [Google Scholar]
- Schell R. F., Chan J. K., LeFrock J. L., Bagasra O. Endemic syphilis: transfer of resistance to Treponema pallidum strain Bosnia A in hamsters with a cell suspension enriched in thymus-derived cells. J Infect Dis. 1980 Jun;141(6):752–758. doi: 10.1093/infdis/141.6.752. [DOI] [PubMed] [Google Scholar]
- Schell R. F., LeFrock J. L., Chan J. K., Bagasra O. LSH hamster model of syphilitic infection. Infect Immun. 1980 Jun;28(3):909–913. doi: 10.1128/iai.28.3.909-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadakuma T., Pierce C. W. Mode of action of a soluble immune response suppressor (SIRS) produced by concanavalin a-activated spleen cells. J Immunol. 1978 Feb;120(2):481–486. [PubMed] [Google Scholar]
- Titus R. G., Weiser R. S. Experimental syphilis in the rabbit: passive transfer of immunity with immunoglobulin G from immune serum. J Infect Dis. 1979 Dec;140(6):904–913. doi: 10.1093/infdis/140.6.904. [DOI] [PubMed] [Google Scholar]
- Wicher V., Wicher K. Cell response in rabbits infected with T. pallidum as measured by the leucocyte migration inhibition test. Br J Vener Dis. 1975 Aug;51(4):240–245. doi: 10.1136/sti.51.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. I. Lymphocyte transformation. Clin Exp Immunol. 1977 Sep;29(3):480–486. [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. II. Inhibition of lymphocyte response to phytohaemagglutinin by serum of T. pallidum-infected rabbits. Clin Exp Immunol. 1977 Sep;29(3):487–495. [PMC free article] [PubMed] [Google Scholar]