Abstract
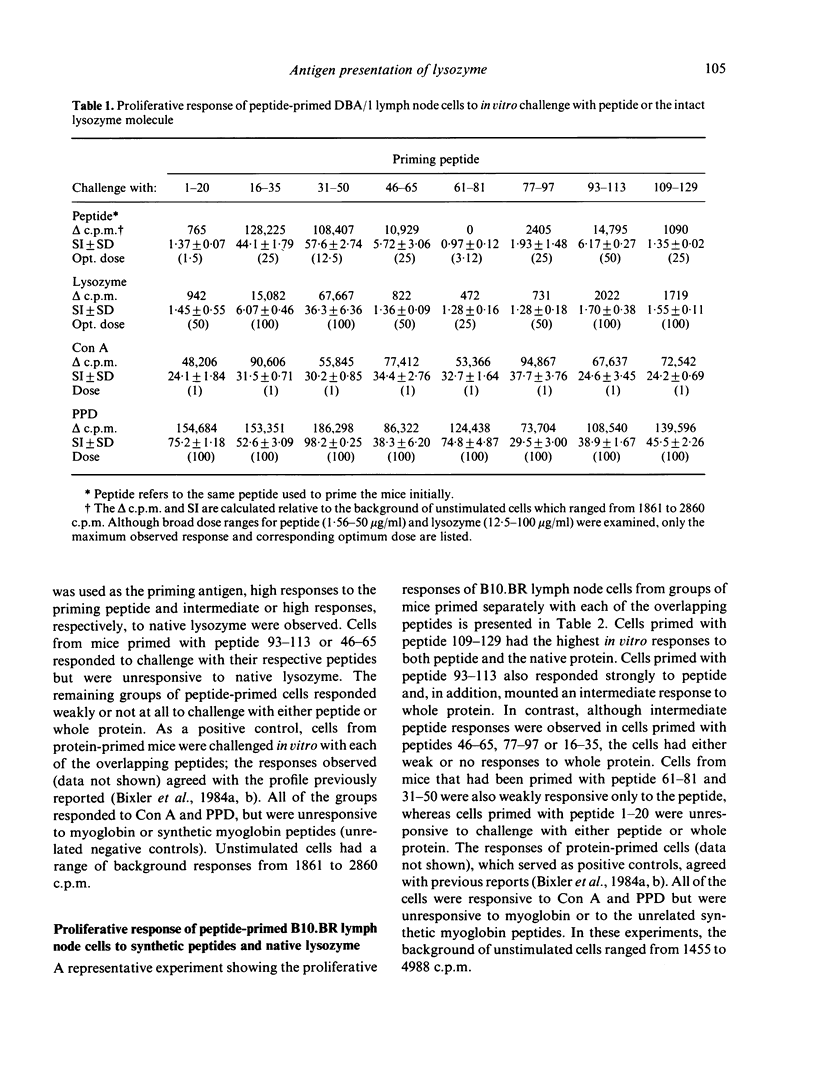
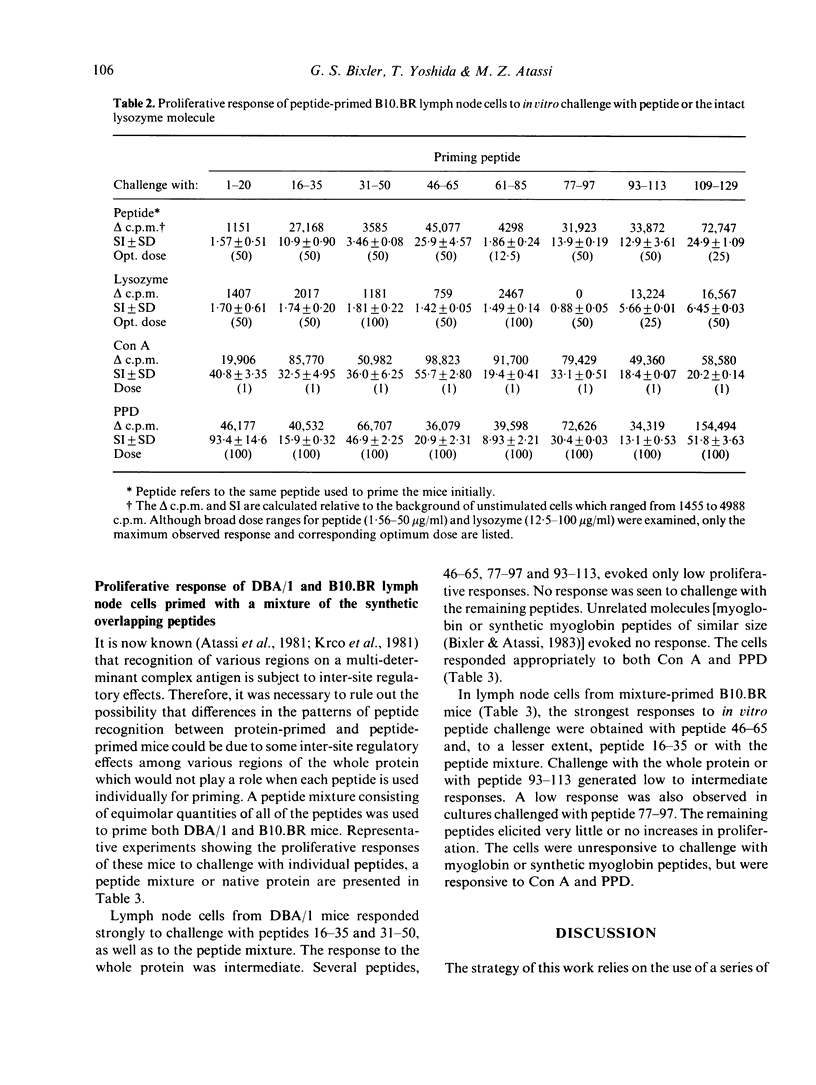
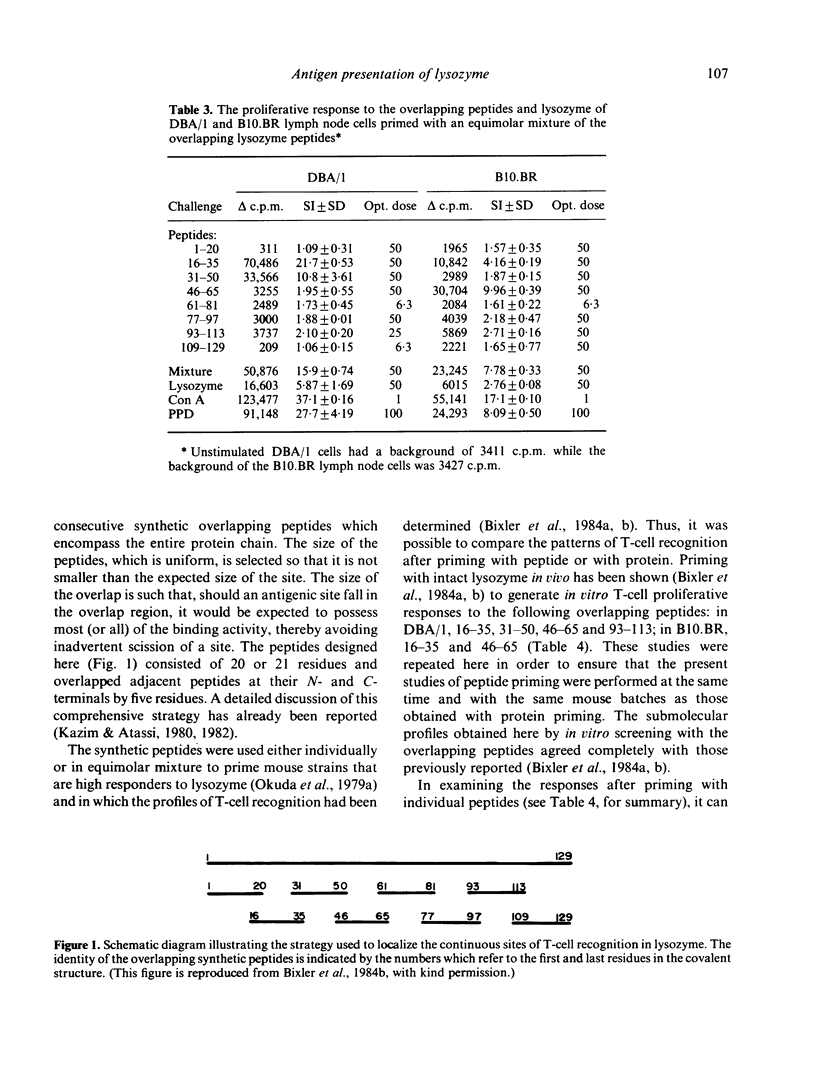
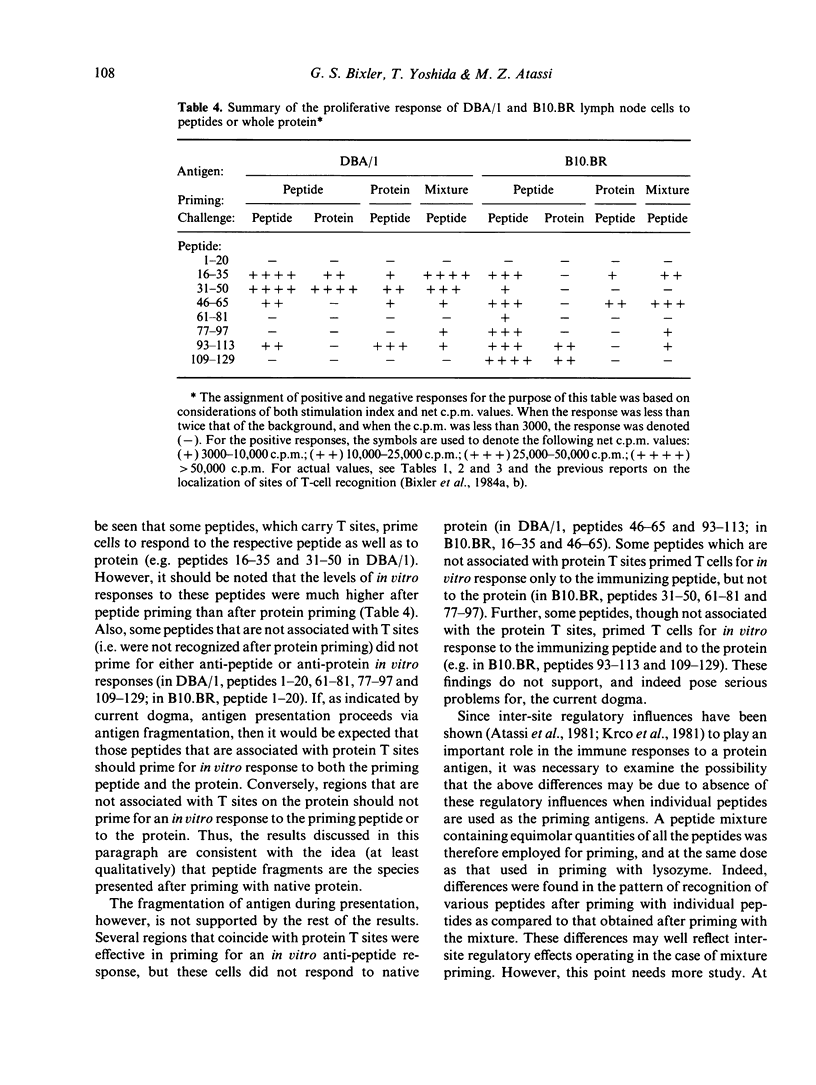
Recently, using synthetic overlapping peptides which encompass the entire protein chain of hen egg lysozyme, the full submolecular profile of continuous regions on the protein recognized by T cells (T sites) was localized. In the present report, we have examined in two mouse strains the proliferative response to peptides and to native protein of lymph node cells from mice primed with synthetic overlapping peptides, either individually or as a mixture. It was found that the pattern of T-cell recognition observed after priming with peptides differs from that obtained when the native protein is used as the immunogen. Some, but not all, of the T-site containing peptides were effective in priming for an anti-lysozyme T-cell response. Several peptides which were highly immunogenic as free synthetic peptides were not associated with any of the known protein T sites. Further, some peptides were effective in priming for T cells that respond in vitro to the priming peptide, but not to the whole protein. If antigen processing proceeds via fragmentation, then only those regions containing T sites would be expected to be effective in priming for a T-cell response to the intact protein. Since this was not found to be the case, it is unlikely that fragmentation of lysozyme is a prerequisite for antigen presentation. Rather, we suggest that the critical aspects in the presentation of a protein antigen predominantly involve recognition of an intact protein, the interaction of which with the cell membrane triggers cellular activating events.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Beller D. I., Braun J., Unanue E. R. The handling of Listeria monocytogenes by macrophages: the search for an immunogenic molecule in antigen presentation. J Immunol. 1984 Jan;132(1):323–331. [PubMed] [Google Scholar]
- Allen P. M., Strydom D. J., Unanue E. R. Processing of lysozyme by macrophages: identification of the determinant recognized by two T-cell hybridomas. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2489–2493. doi: 10.1073/pnas.81.8.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Anderson R. G., Goldstein J. L., Brown M. S. A mutation that impairs the ability of lipoprotein receptors to localise in coated pits on the cell surface of human fibroblasts. Nature. 1977 Dec 22;270(5639):695–699. doi: 10.1038/270695a0. [DOI] [PubMed] [Google Scholar]
- Arnon R., Maron E., Sela M., Anfinsen C. B. Antibodies reactive with native lysozyme elicited by a completely synthetic antigen. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1450–1455. doi: 10.1073/pnas.68.7.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z. Antigenic structure of myoglobin: the complete immunochemical anatomy of a protein and conclusions relating to antigenic structures of proteins. Immunochemistry. 1975 May;12(5):423–438. doi: 10.1016/0019-2791(75)90010-5. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Antigenic structures of proteins. Their determination has revealed important aspects of immune recognition and generated strategies for synthetic mimicking of protein binding sites. Eur J Biochem. 1984 Nov 15;145(1):1–20. doi: 10.1111/j.1432-1033.1984.tb08516.x. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Determination of the entire antigenic structure of native lysozyme by surface-simulation synthesis, a novel concept in molecular recognition. CRC Crit Rev Biochem. 1979;6(4):371–400. doi: 10.3109/10409237909105426. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Lee C. L. Boundary refinement of the lysozyme antigenic site around the disulphide bond 6-127 (site 1) by 'surface-simulation' synthesis. Biochem J. 1978 May 1;171(2):419–427. doi: 10.1042/bj1710419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z., Lee C. L. The precise and entire antigenic structure of native lysozyme. Biochem J. 1978 May 1;171(2):429–434. doi: 10.1042/bj1710429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z. Precise determination of the entire antigenic structure of lysozyme: molecular features of protein antigenic structures and potential of "surface-simulation" synthesis--a powerful new concept for protein binding sites. Immunochemistry. 1978 Dec;15(12):909–936. doi: 10.1016/0161-5890(78)90126-8. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Smith J. A. A proposal for the nomenclature of antigenic sites in peptides and proteins. Immunochemistry. 1978 Aug;15(8):609–610. doi: 10.1016/0161-5890(78)90016-0. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Yokota S., Twining S. S., Lehmann H., David C. S. Genetic control of the immune response to myoglobin. VI. Inter-site influences in T-lymphocyte proliferative response from analysis of cross-reactions of ten myoglobins in terms of substitutions in the antigenic sites and in environmental residues of the sites. Mol Immunol. 1981 Oct;18(10):945–948. doi: 10.1016/0161-5890(81)90017-1. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson R. G., Brown M. S. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981 May;24(2):493–502. doi: 10.1016/0092-8674(81)90340-8. [DOI] [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A. Genetic control of the immune response to mammalian myoglobins in mice I. More than one I-region gene in H-2 controls the antibody response. J Immunol. 1978 Feb;120(2):360–369. [PubMed] [Google Scholar]
- Bixler G. S., Jr, Atassi M. Z. Molecular localization of the full profile of the continuous regions recognized by myoglobin-primed T-cells using synthetic overlapping peptides encompassing the entire molecule. Immunol Commun. 1983;12(6):593–603. doi: 10.3109/08820138309025440. [DOI] [PubMed] [Google Scholar]
- Bixler G. S., Jr, Atassi M. Z. T cell recognition of lysozyme. III. Recognition of the 'surface-simulation' synthetic antigenic sites. J Immunogenet. 1984 Jun-Aug;11(3-4):245–250. doi: 10.1111/j.1744-313x.1984.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Bixler G. S., Jr, Atassi M. Z. T cell recognition of myoglobin. Localization of the sites stimulating T cell proliferative responses by synthetic overlapping peptides encompassing the entire molecule. J Immunogenet. 1984 Oct-Dec;11(5-6):339–353. doi: 10.1111/j.1744-313x.1984.tb00820.x. [DOI] [PubMed] [Google Scholar]
- Bixler G. S., Jr, Yoshida T., Atassi M. Z. T cell recognition of lysozyme. IV. Localization and genetic control of the continuous T cell recognition sites by synthetic overlapping peptides representing the entire protein chain. J Immunogenet. 1984 Oct-Dec;11(5-6):327–337. doi: 10.1111/j.1744-313x.1984.tb00819.x. [DOI] [PubMed] [Google Scholar]
- Bixler G. S., Jr, Yoshida T., Atassi M. Z. T-cell recognition of lysozyme. I. Localization of regions stimulating T-cell proliferative response by synthetic overlapping peptides encompassing the entire molecule. Exp Clin Immunogenet. 1984;1(2):99–111. [PubMed] [Google Scholar]
- Buchmüller Y., Corradin G. Lymphocyte specificity to protein antigens. V. Conformational dependence of activation of cytochrome c-specific T cells. Eur J Immunol. 1982 May;12(5):412–416. doi: 10.1002/eji.1830120510. [DOI] [PubMed] [Google Scholar]
- Chesnut R. W., Colon S. M., Grey H. M. Requirements for the processing of antigens by antigen-presenting B cells. I. Functional comparison of B cell tumors and macrophages. J Immunol. 1982 Dec;129(6):2382–2388. [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Fan J. Y., Carpentier J. L., Gorden P., Van Obberghen E., Blackett N. M., Grunfeld C., Orci L. Receptor-mediated endocytosis of insulin: role of microvilli, coated pits, and coated vesicles. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7788–7791. doi: 10.1073/pnas.79.24.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwing J., Thompson K. Studies on the antigenic properties of egg-white lysozyme. I. Isolation and characterization of a tryptic peptide from reduced and alkylated lysozyme exhibiting haptenic activity. Biochemistry. 1968 Nov;7(11):3888–3892. doi: 10.1021/bi00851a015. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. Evidence for reutilization of surface receptors for alpha-macroglobulin.protease complexes in rabbit alveolar macrophages. Cell. 1980 Jan;19(1):197–205. doi: 10.1016/0092-8674(80)90401-8. [DOI] [PubMed] [Google Scholar]
- Kazim A. L., Atassi M. Z. A novel and comprehensive synthetic approach for the elucidation of protein antigenic structures. Determination of the full antigenic profile of the alpha-chain of human haemoglobin. Biochem J. 1980 Oct 1;191(1):261–264. doi: 10.1042/bj1910261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazim A. L., Atassi M. Z. Structurally inherent antigenic sites. Localization of the antigenic sites of the alpha-chain of human haemoglobin in three host species by a comprehensive synthetic approach. Biochem J. 1982 Apr 1;203(1):201–208. doi: 10.1042/bj2030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krco C. J., Kazim A. L., Atassi M. Z., David C. S. Genetic control of the immune response to haemoglobin. II. Studies using purified alpha-chain and beta-chain as immunogens. J Immunogenet. 1981 Oct;8(5):395–403. doi: 10.1111/j.1744-313x.1981.tb00944.x. [DOI] [PubMed] [Google Scholar]
- Lee C. L., Atassi M. Z. Conformation and immunochemistry of methylated and carboxymethyulated derivatives of lysozyme. Biochemistry. 1973 Jul 3;12(14):2690–2695. doi: 10.1021/bi00738a022. [DOI] [PubMed] [Google Scholar]
- Lee C. L., Atassi M. Z. Delineation of the third antigenic site of lysozyme by application of a novel 'surface-simulation' synthetic approach directly linking the conformationally adjacent residues forming the site. Biochem J. 1976 Oct 1;159(1):89–93. doi: 10.1042/bj1590089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. L., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. Accurate definition of the antigenic site around the disulphide bridge 30-115 (site 3) by 'surface-simulation' synthesis. Biochem J. 1977 Dec 1;167(3):571–581. doi: 10.1042/bj1670571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. L., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. XIX. Definition of the direction and conformational restrictions of the antigenic site around the disulfide bonds 64-80 and 76-94 (site 2) by 'surface-simulation' synthesis. Biochim Biophys Acta. 1977 Dec 20;495(2):354–368. doi: 10.1016/0005-2795(77)90391-9. [DOI] [PubMed] [Google Scholar]
- Lozner E. C., Sachs D. H., Shearer G. M. Genetic control of the immune response to staphylococcal nuclease. I. Ir-Nase: control of the antibody response to nuclease by the Ir region of the mouse H-2 complex. J Exp Med. 1974 May 1;139(5):1204–1214. doi: 10.1084/jem.139.5.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels R. M., Clarke J. A., Harvey M. A., Miller A., Sercarz E. E. Epitope specificity of the T cell proliferative response to lysozyme: proliferative T cells react predominantly to different determinants from those recognized by B cells. Eur J Immunol. 1980 Jul;10(7):509–515. doi: 10.1002/eji.1830100705. [DOI] [PubMed] [Google Scholar]
- Marsh M., Bolzau E., Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983 Mar;32(3):931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers I., Rajewsky K., Shreffler D. C. Ir-LDHB: map position and functional analysis. Eur J Immunol. 1973 Dec;3(12):754–761. doi: 10.1002/eji.1830031204. [DOI] [PubMed] [Google Scholar]
- Merion M., Sly W. S. The role of intermediate vesicles in the adsorptive endocytosis and transport of ligand to lysosomes by human fibroblasts. J Cell Biol. 1983 Mar;96(3):644–650. doi: 10.1083/jcb.96.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Christadoss P. R., Twining S., Atassi M. Z., David C. S. Genetic control of immune response to sperm whale myoglobin in mice. I. T lymphocyte proliferative response under H-2-linked Ir gene control. J Immunol. 1978 Sep;121(3):866–868. [PubMed] [Google Scholar]
- Okuda K., Sakata S., Atassi M. Z., David C. S. Genetic control of the immune response to hen's egg-white lysozyme in mice. I. Antibody and T-lymphocyte proliferative responses to the native protein. J Immunogenet. 1979 Dec;6(6):447–452. doi: 10.1111/j.1744-313x.1979.tb00699.x. [DOI] [PubMed] [Google Scholar]
- Okuda K., Twining S. S., David C. S., Atassi M. Z. Genetic control of immune response to sperm whale myoglobin in mice. II. T lymphocyte proliferative response to the synthetic antigenic sites. J Immunol. 1979 Jul;123(1):182–188. [PubMed] [Google Scholar]
- Pilch P. F., Shia M. A., Benson R. J., Fine R. E. Coated vesicles participate in the receptor-mediated endocytosis of insulin. J Cell Biol. 1983 Jan;96(1):133–138. doi: 10.1083/jcb.96.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role of macrophages in the immune response. Immunol Rev. 1978;40:1–255. [PubMed] [Google Scholar]
- Rosenthal A. S. Regulation of the immune response--role of the macrophage. N Engl J Med. 1980 Nov 13;303(20):1153–1156. doi: 10.1056/NEJM198011133032005. [DOI] [PubMed] [Google Scholar]
- Scibienski R. J., Klingmann V., Leung C., Thompson K., Benjamini E. Recognition of lysozyme by lymphocyte subsets. Adv Exp Med Biol. 1978;98:305–317. doi: 10.1007/978-1-4615-8858-0_16. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer C. J., Ashwell G. Studies on a mammalian hepatic binding protein specific for asialoglycoproteins. Evidence for receptor recycling in isolated rat hepatocytes. J Biol Chem. 1980 Apr 10;255(7):3008–3013. [PubMed] [Google Scholar]
- Straubinger R. M., Hong K., Friend D. S., Papahadjopoulos D. Endocytosis of liposomes and intracellular fate of encapsulated molecules: encounter with a low pH compartment after internalization in coated vesicles. Cell. 1983 Apr;32(4):1069–1079. doi: 10.1016/0092-8674(83)90291-x. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Kojima A., Yaginuma K., Egashira Y. Cell-mediated and humoral immunity in mice: cross reaction between lysozyme and S-carboxymethylated lysozyme studied by a modified footpad test. Jpn J Med Sci Biol. 1975 Feb;28(1):23–35. doi: 10.7883/yoken1952.28.23. [DOI] [PubMed] [Google Scholar]
- Takagaki Y., Hirayama A., Fujio H., Amano T. Antibodies to a continuous region at residues 38-54 of hen egg white lysozyme found in a small fraction of anti-hen egg white lysozyme antibodies. Biochemistry. 1980 May 27;19(11):2498–2505. doi: 10.1021/bi00552a031. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Yoshioka M., Bixler G. S., Jr, Atassi M. Z. Preparation of T-lymphocyte lines and clones with specificities to preselected protein sites by in vitro passage with free synthetic peptides: demonstration with myoglobin sites. Mol Immunol. 1983 Oct;20(10):1133–1137. doi: 10.1016/0161-5890(83)90123-2. [DOI] [PubMed] [Google Scholar]
- Young C. R., Atassi M. Z. T-lymphocyte recognition of sperm-whale myoglobin. Responses of T-cells from mouse strains primed with synthetic peptide carrying antigenic site 5 and relation to antigen presentation. J Immunogenet. 1983 Apr;10(2):151–160. doi: 10.1111/j.1744-313x.1983.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Young J. D., Leung C. Y. Immunochemical studies on lysozyme and carboxymethylated lysozyme. Biochemistry. 1970 Jul 7;9(14):2755–2762. doi: 10.1021/bi00816a001. [DOI] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]


