Abstract
Helicobacter pylori induces activation of mitogen-activated protein kinases (MAPKs). However, its effect on H. pylori-induced apoptosis has not been evaluated. Thus, we examined whether H. pylori-induced extracellular signal-regulated kinase 1 and 2 (ERK1/2) and p38 MAPK activation affects gastric epithelial cell apoptosis and bcl-2 family gene expression, especially in relation to the cagA status of an H. pylori strain. In flow cytometric and oligonucleosome-bound DNA enzyme-linked immunosorbent assay analyses, infection with cagA+ H. pylori strains induced gastric cancer cell apoptosis in AGS cells more prominently than infection with cagA mutants. Activation of ERK1/2 and p38 MAPKs was also more prominent in cagA+ strains. Pretreatment with a MEK inhibitor (PD98059) inhibited ERK1/2 activation and increased H. pylori-induced apoptosis significantly. This increased apoptosis was accompanied by decreased antiapoptotic bcl-2 mRNA expression among bcl-2-related genes (bcl-2, bax, bak, mcl-1, and bcl-XL/S), and the effect was also more prominent in the cagA+ strains. However, the alteration of bcl-2 gene expression was not accompanied by protein level changes. Inhibition of p38 using specific inhibitor SB203580 decreased H. pylori-induced apoptosis but resulted in little alteration of bcl-2-related gene expression. In conclusion, H. pylori-induced ERK1/2 activation, especially by the cagA+ H. pylori strain, may play a protective role against gastric epithelial cell apoptosis partially through maintenance of bcl-2 gene expression.
Helicobacter pylori is a primary etiological agent leading to chronic gastritis and peptic ulcers (29). The organism is associated with gastric cancer in experimental animal models and in epidemiological studies (8, 13, 45). Although the World Health Organization concluded that H. pylori is a definite group I carcinogen (15), the detailed mechanism of carcinogenesis remains unknown. There have been suggestions that H. pylori-induced apoptosis might play an important role in gastric carcinogenesis (48). Apoptosis, i.e., programmed cell death, is a central mechanism for regulating the number of cells in adult tissue and is present as a physiological phenomenon in the normal gastrointestinal tract (12). H. pylori-infected gastric mucosae demonstrate increased epithelial apoptosis, and it may be the stimulation of a compensatory hyperproliferation which causes potentially preneoplastic change in cases of chronic H. pylori infection (16, 26).
The mechanism by which H. pylori induces apoptosis in infected gastric mucosae has not yet been fully elucidated, but several apoptosis-related genes and proteins, such as the Bcl-2 family proteins, Fas-Fas ligand, and p53, may be associated with epithelial cell apoptosis (22, 28, 38). Bcl-2 related proteins can be subdivided into two groups according to their antiapoptotic (e.g., Bcl-2, Mcl-1, and Bcl-XL) or proapoptotic (e.g., Bax, Bak, and Bad) functions (1). Recently, it was suggested that H. pylori induces apoptosis in the gastric epithelium due to both an upregulation of proapoptotic Bax and a downregulation of antiapoptotic Bcl-2 (22). Moreover, in vacuolating cytotoxin (VacA)-transfected cell lines, cotransfection of DNA encoding Bcl-2 blocked mitochondria-dependent caspase-3 activation (9). These results indicate that Bcl-2 may be an important regulator of H. pylori-induced apoptosis.
Mitogen-activated protein kinase (MAPK) signal transduction pathways are members of a family of serine/threonine kinases that are activated by extracellular stimuli. Three main groups of MAPKs, i.e., the extracellular signal-regulated kinases 1 and 2 (ERK1/2), p38 MAPKs, and c-Jun N-terminal kinases are well characterized (10). The ERK1/2 pathway and its upstream kinases are activated by growth factor stimuli and have the effect of transducing the signal to the nucleus and of increasing the level of expression of genes that are related to cellular proliferation (3, 31). The ERK1/2 pathway is also associated with cellular apoptosis (4, 49). Recently, it was suggested that inhibition of ERK1/2 activities causes a downregulation of antiapoptotic homologs such as Bcl-2, Mcl-1, and Bcl-XL and that activation of the pathway functions to protect cells from apoptosis (2, 7). H. pylori can activate the three main MAPK signaling pathways (19). However, it has not been established that H. pylori-induced ERK1/2 and p38 MAPK activation affects the apoptosis of epithelial cells and bcl-2 family gene expression.
The cytotoxin-associated gene A (cagA) codes for an immunodominant antigen and is closely associated with the cag pathogenicity islands (PAI) that encode disease-associated virulence proteins (5). Infection with cagA+ H. pylori strains is more closely associated with peptic ulceration and gastric cancer than infection with cagA mutants (33, 46). Also, the isogenic mutant strain of specific cag region genes reduces NF-κB activation and interleukin-8 gene transcription (5, 11, 41). cagA+ H. pylori strains are more potent in inducing gastric epithelial cell MAPK phosphorylation (19). However, whether the cagA status of an H. pylori strain affects gastric epithelial apoptosis remains controversial (27, 34, 37). Whether cagA status affects bcl-2 gene expression is also unknown.
The aim of this study was to determine whether H. pylori-induced ERK1/2 activation is associated with gastric epithelial cell apoptosis and whether its effect on bcl-2 family gene expression is dependent on the cagA status of the H. pylori strain.
MATERIALS AND METHODS
H. pylori strains and culture.
H. pylori clinical strains were isolated from gastric mucosal biopsies obtained from patients with gastric or duodenal ulcers at Seoul National University Hospital, Seoul, Korea. H. pylori was cultured under a microaerophilic atmosphere (5% O2, 10% CO2, 85% N2) at 37°C. After initial isolation of a single colony from each biopsy specimen, the H. pylori strains were subcultured and preserved at −70°C in brucella broth (Difco Laboratories, Detroit, Mich.) supplemented with 15% (vol/vol) glycerol. These preparations were thawed and subcultured, within a span of 10 generations, on solid agar plates containing 5% sheep blood and 0.024% yeast extract. The presence of cagA and vacuolating cytotoxin activity was determined as previously described (20).
Cell culture and H. pylori infection.
AGS gastric adenocarcinoma cells (ATCC CRL 1739) were grown in Ham's F-12 medium (Sigma, St. Louis, Mo.) (pH 7.4) supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% fetal bovine serum (HyClone Laboratories, Logan, Utah). Cell cultures were maintained at 37°C and 5% CO2 in a humidified incubator. Cell culture experiments were conducted in 6-well polypropylene tissue culture plates (Corning Costar, Cambridge, Mass.) 48 h after the antibiotics were removed. For the infection, the bacteria were harvested in phosphate-buffered saline (PBS) (pH 7.4) by using sterile cotton swabs and diluted to a final concentration corresponding to a cell-to-bacteria ratio of 1:100. An uninfected control was included in each experiment.
Pretreatment of AGS cells with specific MAPK inhibitors.
The specific MEK1/2 inhibitor PD98059 (Calbiochem, La Jolla, Calif.) and specific p38 MAPK inhibitor SB203580 (Calbiochem) were purchased, and stock solutions were prepared in dimethyl sulfoxide. AGS cells were treated with PD98059 or SB203580, at the indicated final concentration, 1 h before exposure to H. pylori. Control cells without inhibitors were treated with dimethyl sulfoxide alone.
Western blot analysis of phosphospecific MAPK activation.
AGS cells were grown to confluence on 6-well culture plates and maintained in a serum-free medium for 6 h before the experiment. The monolayer of the AGS cells was washed three times with PBS and lysed with sodium dodecyl sulfate (SDS) buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 50 mM dithiothreitol, 0.1% bromophenol blue). Samples were sonicated for 10 s to shear the DNA and to reduce viscosity, heated to 100°C for 5 min, microcentrifuged for 5 min after cooling on ice, and then loaded onto an SDS-10% polyacrylamide gel. After running the gel, the proteins were transferred onto nitrocellulose membranes (Hybond ECL; Amersham, Little Chalfont, United Kingdom). The membranes were blocked for 1 h at room temperature with a 5% solution of nonfat dry milk in Tris-buffered saline (pH 7.4). This was followed by 1 h of incubation at room temperature with the phosphospecific MAPK antibodies diluted 1/1000 in a dilution buffer (containing Tris-buffered saline and 0.1% Tween-20 with 1% nonfat dry milk). The membranes were then washed three times with Tris-buffered saline and incubated at room temperature for 1 h with peroxidase-conjugated goat anti-rabbit antibody (1/1000 dilution; Vector Laboratories, Burlingame, Calif.). The antigen-antibody complexes were detected with an enhanced chemiluminescence detection kit (ECL; Amersham). Each sample was probed with nonphosphospecific MAPK antibodies to indicate the equivalent protein amount in each lane. Phosphospecific p44/p42 MAPK antibody (New England Biolabs, Beverly, Mass.) was used to detect phosphorylated ERK1/2. Phosphospecific p38 MAPK antibody (New England Biolabs) was used to detect phosphorylated p38 MAPK. Nonphosphospecific antibodies to ERK1/2 and p38 MAPK were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.).
Assay for apoptosis in gastric epithelial cell lines.
Flow cytometric analysis of apoptotic cells was performed using an Annexin V-FITC apoptosis detection kit (Pharmingen, San Diego, Calif.). In brief, adherent AGS cells in 6-well culture plates were harvested by trypsin treatment after PBS washing followed by resuspension in a binding buffer at a concentration of 106 cells per ml. Fluorescein isothiocyanate (FITC)-conjugated annexin V and propidium iodide were added to 105 cells, after which the cells were incubated for 15 min at room temperature in a dark chamber. The apoptotic cell proportion was quantified a flow cytometry apparatus (FACStar plus; Becton Dickinson, San Jose, Calif.).
DNA fragmentation was quantified by means of a sandwich enzyme-linked immunosorbent assay (ELISA), using a commercially available kit (Cell Death Detection ELISAplus; Roche, Mannheim, Germany) as described in the manufacturer's manual. In brief, AGS cells, cultured in 6-well culture plates, were directly lysed with a lysis buffer, after which the cytosolic oligonucleosome was quantified using biotin-labeled mouse monoclonal anti-histone antibody as the capturing antibody, peroxidase-conjugated mouse monoclonal anti-DNA antibody as the detecting antibody, and ABTC (2,2′-azino-di[3-ethylbenzthiazolin-sulfonate]) as the developing agent. The relative increase in nucleosomes in the cytoplasm was expressed as an enrichment factor, which was calculated as the ratio of the specific absorbance of lysates at 405 nm to that of untreated cells.
RNA extraction and reverse transcription-PCR (RT-PCR) analysis of bcl-2 family genes.
Total cellular RNA was extracted from AGS cells by the acid guanidinium-phenol-chloroform method (Trizol; Gibco BRL, Gaithersburg, Md.). Reverse transcription was performed as described in a previous report (17). A cDNA PCR procedure was performed using specific primers for β-actin and bcl-2 family genes as described in a previous report (21). PCR amplification was performed in a thermal cycler (GenAmp PCR system 9600; Perkin-Elmer Cetus, Norwalk, Conn.). The amplification profile consisted of 33 cycles of 45 s of denaturation at 95°C and 1 min 45 s of annealing and extension at 65°C for the β-actin gene, a housekeeping gene. For the bcl-2, bak, and mcl-1 genes, the amplification profile consisted of 40 cycles of 1 min denaturation at 94°C, 1 min of annealing at 60°C, and 1 min of extension at 72°C; 33 cycles of this same profile were used for the bax gene. The bcl-XL/S gene amplification profile consisted of 35 cycles of 30 s of denaturation at 94°C, 30 s of annealing at 55°C, and 30 s of extension at 72°C. PCR products were separated on a 2% NuSieve agarose gel (FMC Bioproducts, Rockland, Maine) and stained with ethidium bromide.
Western blot analysis of bcl-2 family genes.
Subconfluent monolayers of AGS cells were cocultured with H. pylori for 16 h, and then cell lysates were prepared in an ice-cold lysis buffer (20 mM Tris [pH 7.4], 150 mM NaCl, 0.5% NP-40, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml each of aprotinin and leupeptin). The protein concentration was measured using the Bradford assay (Bio-Rad, Hercules, Calif.). A total of 20 μg of each sample was electrophoresed in the SDS-10% polyacrylamide gel and subjected to electroblotting on a nitrocellulose membrane (Hybond ECL; Amersham). After overnight incubation at 4°C in a blocking solution containing 5% bovine serum albumin, blots were incubated with primary antibodies (anti-Bcl-2 monoclonal antibody, anti-Bcl-X, anti-Mcl-1, anti-Bax, and anti-Bak polyclonal antibody; Santa Cruz Biotechnology) in 1% bovine serum albumin-Tris-buffered saline-0.1% Tween-20 for 2 h at room temperature. Blots were then incubated with horseradish peroxidase-conjugated immunoglobulin G (Promega, Madison, Wis.) for 1 h at room temperature and developed using an enhanced chemiluminescence detection kit (ECL; Amersham).
Statistical analysis.
Each figure shows the results of experiments repeated at least three times. All statistical analyses were performed using the unpaired Student's t test. A P value of <0.05 was considered statistically significant.
RESULTS
H. pylori-induced ERK1/2 and p38 MAPK activation.
A cytotoxin-positive cagA+ H. pylori clinical strain induced ERK1/2 and p38 MAPK activation. The level of phosphorylation of ERK1/2 increased at 30 min and peaked at 90 min after H. pylori infection (Fig. 1A). The level of phosphorylation of p38 increased at 15 min and peaked at 45 min after infection. Control cells which were not stimulated by H. pylori showed undetectable levels of phosphorylated ERK1/2 and phosphorylated p38. Phosphorylation of ERK1/2 and p38 lasted for 4 h after H. pylori infection. The intracellular levels of total ERK1/2 and p38 remained constant throughout each period.
FIG. 1.
H. pylori-induced ERK1/2 and p38 MAPK activation. AGS gastric epithelial cells were stimulated by H. pylori at a cell-to-bacterium ratio of 1:100. (A) Cell lysates were prepared at the designated time points after H. pylori strain 99 (cagA+, cytotoxin positive) infection. Western blot analysis was performed for phosphorylated ERK1/2 (P-ERK1/2), total ERK1/2, phosphorylated p38 (P-p38), and total p38. (B) AGS gastric epithelial cells were stimulated with H. pylori cagA mutants (strains 28 and 45) and cagA+ strains (strains 92 and 99) with or without pretreatment by MEK1/2 inhibitor PD98050 (20 μM) for 1 h. Cell lysates were prepared at 90 min after H. pylori infection. Western blot analysis was performed for phosphorylated ERK1/2 (upper panel) and total ERK1/2 (lower panel). (C) AGS gastric epithelial cells were stimulated with H. pylori cagA mutants (strains 28 and 45) and cagA+ strains (strains 92 and 99) with or without pretreatment by p38 inhibitor SB203580 (10 μM) for 1 h. Western blot analysis was performed for phosphorylated p38 (upper panel) and total p38 (lower panel). Representative data of three independent experiments are shown.
To investigate whether the cagA status of the H. pylori strain affects MAPK activation capacity, we performed the experiments with H. pylori strains carrying or not carrying the cagA gene. The cagA+ H. pylori strains (strains 92 and 99) induced more-potent phosphorylation of ERK1/2 than the H. pylori cagA mutants (strains 28 and 45). Pretreatment with the specific inhibitor of MEK1/2 (PD98059) abolished ERK1/2 activation (Fig. 1B). Similarly, the cagA+ H. pylori strain induced more-potent phosphorylation of p38 and pretreatment with SB203580 reduced p38 activation in both the cagA+ strains and cagA mutants (Fig. 1C).
Increased H. pylori-induced apoptosis by inhibition of the ERK1/2 pathway.
Monolayers of AGS gastric epithelial cells were cocultured with H. pylori strain 99 (cagA+, cytotoxin positive), and apoptosis was measured by flow cytometric analysis. Figure 2 shows that H. pylori infection of AGC cells increased early apoptotic cell proportions (lower right quadrant) to 18% compared with 5.7% for the uninfected control. The late apoptotic cell proportion (upper right quadrant) was also increased to 15% compared with 2.0% for the uninfected control.
FIG. 2.
H. pylori-induced apoptosis in AGS gastric epithelial cells. Subconfluent monolayers of AGS gastric epithelial cells were cultured without or with H. pylori strain 99 (cagA+, cytotoxin positive). AGS cells were harvested at 16 h after H. pylori infection and incubated with FITC-conjugated annexin V (AV) and propidium iodide (PI) double staining. Flow cytometric analysis was performed, and the data shown are representative of three separate experiments. The lower right quadrants represent early apoptotic cells that were stained by annexin V but not by propidium iodide. The upper right quadrants represent late apoptotic cells that were stained by both annexin V and propidium iodide.
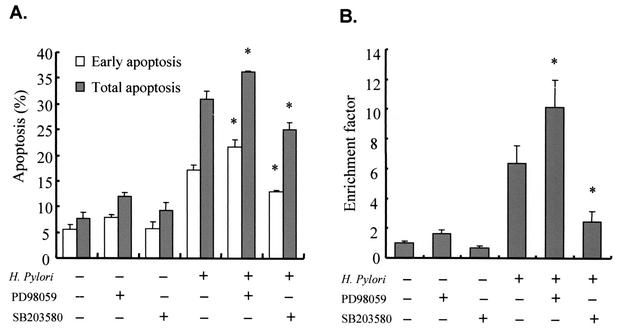
To investigate whether MAPK inhibition affects H. pylori-induced AGS cell apoptosis, we pretreated PD98059 or SB203580 for 1 h before H. pylori infection. In uninfected AGS cells, inhibition of the ERK1/2 pathway slightly increased apoptosis but inhibition of the p38 pathway did not affect apoptosis (Fig. 3A). When the AGS cells were cocultured with a cagA+ H. pylori strain, inhibition of the ERK1/2 pathway augmented H. pylori-induced apoptosis, as measured by flow cytometric analysis. Apoptosis analysis by oligonucleosome-bound DNA ELISA also confirmed that inhibition of the ERK1/2 pathway further augmented apoptosis (Fig. 3B). Inhibition of p38 MAPK resulted in reduction of H. pylori-induced apoptosis (Fig. 3).
FIG. 3.
Augmentation of H. pylori-induced apoptosis by inhibition of ERK1/2 pathway. (A) H. pylori strain 99 (cagA+, cytotoxin positive) and a subconfluent monolayer of AGS cells were cocultured for 16 h with or without pretreatment of PD98059 (20 μM) or SB203580 (10 μM). Data represent early and total apoptotic cell proportions measured by flow cytometric analysis after staining with FITC-conjugated annexin V and propidium iodide. (B) Apoptosis of AGS cells was assessed by a cell death detection ELISA. Values refer to DNA fragmentation as measured by the enrichment factor. Values are expressed as means ± standard deviations of a triplicate experiment and are representative of three separate experiments. *, P < 0.05 (compared with results for H. pylori infection alone).
Effect of MAPK inhibitors on apoptosis-related bcl-2 family gene expression.
bcl-2, bax, bak, and bcl-XL/S mRNA was constitutively expressed in AGS cells. Among them, mRNA expression of the bcl-2 gene was downregulated by pretreatment with PD98059, whereas that of bcl-XL/S, bax, and bak was not changed (Fig. 4). mRNA of mcl-1 was not expressed constitutively and was not induced by H. pylori infection or pretreatment with PD98059. Inhibition of p38 MAPK did not alter bcl-2 family gene expression.
FIG. 4.
Decreased bcl-2 gene expression by inhibition of the ERK1/2 pathway. AGS epithelial cells were cocultured with H. pylori strain 99 (cagA+, cytotoxin positive) with or without pretreatment by MEK1/2 inhibitor PD98050 (20 μM) or p38 inhibitor SB203580 (10 μM) for 1 h. After 6 h of coculture, total cellular RNA was extracted and RT-PCR was performed for bcl-2 family genes. PCR products were separated on a 2% NuSieve agarose gel and stained with ethidium bromide.
cagA status of H. pylori strains and apoptosis.
We investigated whether H. pylori cagA status affects H. pylori-induced apoptosis. Flow cytometric analysis shows that the H. pylori clinical cagA mutants (strains 28 and 45) induced apoptosis to a lesser extent than the cagA+ strains (strains 44, 92, and 99). Pretreatment with PD98059 at a concentration of 20 μM augmented the apoptosis that was induced by each H. pylori strain (Fig. 5A). Oligonucleosome-bound DNA ELISAs showed similar results (Fig. 5B). Taken together, these results show that the cagA+ H. pylori strain induced more apoptosis and that this effect was augmented by blocking of the ERK1/2 pathway.
FIG. 5.
Increased apoptosis by cagA+ H. pylori strains. AGS gastric epithelial cells were infected with H. pylori cagA mutants (strains 28 and 45) or cagA+ strains (strains 44, 92, and 99). H. pylori and a subconfluent monolayer of AGS cells were cocultured for 16 h with or without pretreatment by PD98059 (20 μM). (A) Early apoptotic cell proportions measured by flow cytometric analysis after staining with FITC-conjugated annexin V and propidium iodide. (B) Apoptosis of AGS cells was assessed by means of cell death detection ELISA. Values refer to DNA fragmentation as measured by the enrichment factor. Values are means ± standard deviations of triplicate experiments, and representative data of three independent experiments were shown. *, P < 0.05 (compared with each result for cells without PD98059 treatment).
Effect of ERK1/2 inhibition on bcl-2 expression according to the cagA status of H. pylori strains.
Constitutively expressed bcl-2, bax, bak, and bcl-XL/S mRNA levels were not affected by the cagA status of H. pylori. However, bcl-2 mRNA expression was downregulated when the ERK1/2 pathway was inhibited by pretreatment with PD98059. The level of downregulation was more prominent in the case of the cagA+ strains (strains 44, 92, and 99) than with the cagA mutants (strains 28 and 45) (Fig. 6).
FIG. 6.
Decreased bcl-2 gene expression by the inhibition of the ERK1/2 pathway, especially in cagA+ H. pylori strains. AGS gastric epithelial cells were infected with H. pylori cagA mutants (strains 28 and 45) or cagA+ strains (strains 44, 92, and 99) with or without pretreatment by PD98059 (20 μM). After 6 h of coculturing, total cellular RNA was extracted and RT-PCR was performed for bcl-2-related genes. PCR products were separated on a 2% NuSieve agarose gel and stained with ethidium bromide. Representative data of three separate experiments are shown.
Bcl-2 protein was not constitutively detected in uninfected cells. The protein was detected in H. pylori-stimulated cells, especially when infected by cagA+ strains (Fig. 7). Considering its effect on the downregulation of the bcl-2 gene, we expected that pretreatment with PD98059 would decrease Bcl-2 protein expression when stimulated by the cagA+ strain. Unexpectedly, however, Bcl-2 protein levels were not affected by pretreatment with PD98059.
FIG. 7.
cagA+ H. pylori strain-induced Bcl-2 protein expression. AGS gastric epithelial cells were stimulated with H. pylori cagA mutants (strains 28 and 45) and cagA+ strains (strains 92 and 99) at a cell-to-bacterium ratio of 1:100, with or without pretreatment by PD98059 (20 μM). Cell lysates were prepared 16 h after H. pylori infection. Western blot analysis was performed for Bcl-2 family proteins. These experiments were performed on three occasions, and representative blots are shown.
DISCUSSION
H. pylori induces apoptosis in gastric epithelial cells in cell culture experiments (6, 35, 38, 44). In vivo studies have also shown that H. pylori infection is associated with increased gastric epithelial apoptosis, which returns to a normal level after eradication of the organism (16, 22, 26). Treatment of gastric epithelial cells with tumor necrosis factor alpha, gamma interferon, or Fas ligand markedly increased H. pylori-induced apoptosis compared to coincubation with H. pylori alone (38, 44). These results suggest that several signal transduction pathways affect apoptosis induced by H. pylori infection. The ERK1/2 pathway is mainly associated with growth factor stimuli, which cause the signal to be transduced into the nucleus and increase the expression levels of the genes that are involved in cellular proliferation and differentiation (10). Inhibition of ERK1/2 is associated with the induction of apoptosis in human chondrocytes and in growth-factor-deprived neuronal cells (40, 49). Inhibition of this pathway also sensitizes the cells to apoptotic signaling from FasR and tumor necrosis factor-related apoptosis-inducing ligand receptor and potentiated apoptotic effects of the sulindac metabolite in colon cancer cells (36, 43). ERK1/2 activation also provides protection against DNA damage and apoptosis in hyperoxic rat alveolar epithelial cells (4). All things considered, the ERK/MEK pathway may play a major regulatory role for apoptosis in a variety of cell lines. Our result, showing that inhibition of the ERK1/2 pathway augmented H. pylori-induced apoptosis in gastric epithelial cells, was also consistent with the results of those in vitro studies and points to the protective role of ERK1/2 activation in preventing H. pylori-induced apoptosis.
The underlying mechanism by which inhibition of the ERK/MEK pathway increases H. pylori-induced apoptosis is still uncertain, but several downstream pathways that might affect H. pylori-induced apoptosis have been reported. H. pylori-induced ERK/MEK activation is associated with cyclin D1 gene expression, which is an important regulator of cell cycles (14), and with the expression of proto-oncogene c-fos and the transactivation of the transcriptional factor AP-1 (24, 25) as well as with the induction of hHDC (histidine decarboxylase) promoter transactivation activity (47). However, the relationship between H. pylori-induced ERK1/2 activation and apoptosis-related bcl-2-related gene expression has yet to be elucidated. It was reported that H. pylori-induced apoptosis was accompanied by an increased expression of Bak, a proapoptotic protein, while there was little change in the expression of other Bcl-2 family proteins (6). In an in vivo study, H. pylori infection was also found to be associated with the upregulation of Bax mRNA and protein and the suppressed expression of Bcl-2 mRNA and protein (22). These findings suggest that overexpression of proapoptotic proteins such as Bak and Bax, and underexpression of antiapoptotic proteins such as Bcl-2, plays an important role in H. pylori-induced apoptosis. The activation of the ERK/MAPK pathway is associated with bcl-2 family gene and protein expression. Fibroblast growth factor 2 supports the survival of retinal neuronal cells by upregulating or maintaining bcl-XL and bcl-2 expression via ERK-dependent pathway (7). Also, in the pancreatic cell line, inhibition of ERK1/2 activation was found to be associated with downregulation of antiapoptotic homologs Bcl-2, Mcl-1, and Bcl-XL; thus, activation of the ERK1/2 pathway functions to protect tumor cells from apoptosis (2). Moreover, maturation signals in thymocyte are transduced by the ERK/MAPK signal pathway and upregulate expression of the antiapoptotic protein Bcl-2 (23). Our result shows that the increased apoptosis caused by the inhibition of ERK1/2 activation is accompanied by decreased bcl-2 mRNA expression. The protective role of H. pylori-induced ERK1/2 activation against apoptosis seems to be mediated by maintenance of bcl-2 gene expression.
The question of whether the cagA status of the H. pylori strain is associated with its potential to induce apoptosis remains controversial. It has been suggested that cagA+ strains are associated with an increase in proliferation that is not accompanied by an increase in apoptosis (34, 37). These results support the hypothesis that the cagA+ H. pylori strains predispose to gastric cancer by causing an imbalance between epithelial proliferation and apoptosis. However, this hypothesis has been challenged by an in vitro study that found no association between cagA expression and apoptosis (42) and by in vivo evidence that gastric epithelial apoptosis is increased in duodenal ulcer patients, who are usually infected with cagA+ strains (22, 26). A recent in vivo study also argued against the hypothesis, showing that apoptosis is only significantly increased in patients with cagA+ strains (27), a result that is in accordance with in vitro cell culture studies (35, 38). In the present study, our results show that cagA+ strains induce more apoptosis than the cagA mutant in gastric epithelial cells.
The effect of the cagA status of an H. pylori strain on MAPK activation has also been investigated in several studies. H. pylori-induced MAPK activation has been reported to be more potent in the case of cagA+ H. pylori strains than in that of cagA mutants (19). Meyer-ter-Vehn et al. showed that H. pylori strains carrying intact cag PAI induce activation of the ERK1/2 pathway, resulting in Elk-1 phosphorylation and increased transcription of the proto-oncogene c-fos, results which were abolished in the case of the cagA mutant (24). Mitsuno et al. also showed that H. pylori with an intact cag PAI is a prerequisite for the onset of intracellular signaling leading to AP-1 activation through the ERK1/2 pathway (25). However, a recent study contradicted these results, showing that H. pylori-induced ERK/MEK activation was independent of H. pylori cagA gene expression, because cagA PAI knockout H. pylori mutants also stimulated activation of MEK1/2 and ERK1/2 (47). Which factor or factors are responsible for this discrepancy remains uncertain; however, the results of our present study support the former results indicating that cagA status is associated with the ability to induce ERK/MEK activation.
In the present study, inhibition of the ERK1/2 pathway decreased bcl-2 mRNA expression, and this was found to be more prominent in the case of cagA+ strains. We speculate that bcl-2 mRNA downregulation itself is not the major factor in H. pylori-induced apoptosis, because epithelial cells cocultured with H. pylori alone showed little change in bcl-2 mRNA expression. Rather, ERK1/2 activation by cagA+ strains probably maintains bcl-2 mRNA expression and plays a protective role in the prevention of apoptosis. Unexpectedly, however, Bcl-2 protein levels were increased with H. pylori infection, irrespective of the level of MEK/ERK inhibition. These contradictory results could not be fully explained. However, it has been reported that there may be a discrepancy between Bcl-2 protein levels and mRNA levels and that posttranscriptional modulation might play an important role (22). Also, it was recently reported that MEK/ERK signaling is critical in the upregulation of the antiapoptotic proteins Bcl-XL and Bcl-2 and that this upregulation occurred at the translational rather than the transcriptional level (32). The detailed mechanism behind this dissociation between the upregulation of the antiapoptotic Bcl-2 protein and the downregulation of bcl-2 mRNA expression needs further investigation.
The p38 MAPK signaling pathway is also activated by a variety of extracellular stimuli, and the activation of this pathway results in diverse functional outcomes which seem to be dependent on both stimulus and cell type (30). Activation of the p38 MAPK is known to be associated with the induction of apoptosis in growth-factor-deprived pheochromocytoma cells (49), in sodium-salicylate-treated fibroblast cells (39), and in glutamate-treated cerebellar granule cells (18). Also, these studies showed that inhibition of the p38 MAPK by SB203580 led to apoptosis being blocked in these cases. Similarly, in the present study, we found both that H. pylori induced p38 MAPK activation and that inhibition of p38 by the specific inhibitor decreased H. pylori-induced apoptosis (Fig. 3). However, inhibition of p38 MAPK caused little alteration in mRNA expression (Fig. 4) or in the protein levels of the Bcl-2 family members (data not shown). Thus, we speculate that p38 activation contributes to H. pylori-induced apoptosis by some mechanism other than that involving bcl-2-related gene and protein regulation and suggest that this should be evaluated in further studies.
In conclusion, H. pylori-induced ERK1/2 activation, especially by the cagA+ H. pylori strain, plays a protective role against gastric epithelial cell apoptosis through maintenance of bcl-2 gene expression. Further studies are needed to address the details of the mechanism of Bcl-2 posttranscriptional modulation and to elucidate the mechanism by which the p38 pathway affects H. pylori-induced apoptosis.
Acknowledgments
This study was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (00-PJ1-PG1-CH02-0001).
Editor: J. D. Clements
REFERENCES
- 1.Adams, J. M., and S. Cory. 1998. The Bcl-2 protein family: arbiters of cell survival. Science 281:1322-1326. [DOI] [PubMed] [Google Scholar]
- 2.Boucher, M. J., J. Morisset, P. H. Vachon, J. C. Reed, J. Laine, and N. Rivard. 2000. MEK/ERK signaling pathway regulates the expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of human pancreatic cancer cells. J. Cell. Biochem. 79:355-369. [PubMed] [Google Scholar]
- 3.Brunet, A., D. Roux, P. Lenormand, S. Dowd, S. Keyse, and J. Pouyssegur. 1999. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 18:664-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley, S., B. Driscoll, L. Barsky, K. Weinberg, K. Anderson, and D. Warburton. 1999. ERK activation protects against DNA damage and apoptosis in hyperoxic rat AEC2. Am. J. Physiol. 277:L159-L166. [DOI] [PubMed] [Google Scholar]
- 5.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, G., E. M. Sordillo, W. G. Ramey, J. Reidy, P. R. Holt, S. Krajewski, J. C. Reed, M. J. Blaser, and S. F. Moss. 1997. Apoptosis in gastric epithelial cells is induced by Helicobacter pylori and accompanied by increased expression of BAK. Biochem. Biophys. Res. Commun. 239:626-632. [DOI] [PubMed] [Google Scholar]
- 7.Desire, L., Y. Courtois, and J. C. Jeanny. 2000. Endogenous and exogenous fibroblast growth factor 2 support survival of chick retinal neurons by control of neuronal neuronal bcl-xL and bcl-2 expression through a fibroblast growth factor receptor 1- and ERK-dependent pathway. J. Neurochem. 75:151-163. [DOI] [PubMed] [Google Scholar]
- 8.Eslick, G. D., L. L. Lim, J. E. Byles, H. H. Xia, and N. J. Talley. 1999. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am. J. Gastroenterol. 94:2373-2379. [DOI] [PubMed] [Google Scholar]
- 9.Galmiche, A., J. Rassow, A. Doye, S. Cagnol, J. C. Chambard, S. Contamin, V. de Thillot, I. Just, V. Ricci, E. Solcia, E. Van Obberghen, and P. Boquet. 2000. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 19:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrington, T. P., and G. L. Johnson. 1999. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 11:211-218. [DOI] [PubMed] [Google Scholar]
- 11.Glocker, E., C. Lange, A. Covacci, S. Bereswill, M. Kist, and H. L. Pahl. 1998. Proteins encoded by the cag pathogenicity island of Helicobacter pylori are required for NF-κB activation. Infect. Immun. 66:2346-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall, P. A., P. J. Coates, B. Ansari, and D. Hopwood. 1994. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J. Cell Sci. 107:3569-3577. [DOI] [PubMed] [Google Scholar]
- 13.Helicobacter and Cancer Collaborative Group. 2001. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 49:347-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirata, Y., S. Maeda, Y. Mitsuno, M. Akanuma, Y. Yamaji, K. Ogura, H. Yoshida, Y. Shiratori, and M. Omata. 2001. Helicobacter pylori activates the cyclin D1 gene through mitogen-activated protein kinase pathway in gastric cancer cells. Infect. Immun. 69:3965-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Agency for Research on Cancer. 1994. Evaluation of carcinogenic risks to humans. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr. Eval. Carcinog. Risks Hum. 61:1-241. [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, N. L., P. T. Shannon, E. Cutz, H. Yeger, and P. M. Sherman. 1997. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am. J. Pathol. 151:1695-1703. [PMC free article] [PubMed] [Google Scholar]
- 17.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawasaki, H., T. Morooka, S. Shimohama, J. Kimura, T. Hirano, Y. Gotoh, and E. Nishida. 1997. Activation and involvement of p38 mitogen-activated protein kinase in glutamate-induced apoptosis in rat cerebellar granule cells. J. Biol. Chem. 272:18518-18521. [DOI] [PubMed] [Google Scholar]
- 19.Keates, S., A. C. Keates, M. Warny, R. M. Peek, P. G. Murray, and C. P. Kelly. 1999. Differential activation of mitogen-activated protein kinases in AGS gastric epithelial cells by cag+ and cag− Helicobacter pylori. J. Immunol. 163:5552-5559. [PubMed] [Google Scholar]
- 20.Kim, J. S., H. C. Jung, J. M. Kim, I. S. Song, and C. Y. Kim. 1998. Interleukin-8 expression by human neutrophils activated by Helicobacter pylori soluble proteins. Scand. J. Gastroenterol. 33:1249-1255. [DOI] [PubMed] [Google Scholar]
- 21.Kim, J. S., J. M. Kim, H. C. Jung, I. S. Song. 2001. Caspase-3 activity and expression of Bcl-2 family in human neutrophils by Helicobacter pylori water-soluble proteins. Helicobacter 6:207-215. [DOI] [PubMed] [Google Scholar]
- 22.Konturek, P. C., P. Pierzchalski, S. J. Konturek, H. Meixner, G. Faller, T. Kirchner, and E. G. Hahn. 1999. Helicobacter pylori induces apoptosis in gastric mucosa through an upregulation of Bax expression in humans. Scand. J. Gastroenterol. 34:375-383. [DOI] [PubMed] [Google Scholar]
- 23.McKean, D. J., C. J. Huntoon, M. P. Bell, X. Tai, S. Sharrow, K. E. Hedin, A. Conley, and A. Singer. 2001. Maturation versus death of developing double-positive thymocytes reflects competing effects on Bcl-2 expression and can be regulated by the intensity of CD28 costimulation. J. Immunol. 166:3468-3475. [DOI] [PubMed] [Google Scholar]
- 24.Meyer-ter-Vehn, T., A. Covacci, M. Kist, and H. L. Pahl. 2000. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J. Biol. Chem. 275:16064-16072. [DOI] [PubMed] [Google Scholar]
- 25.Mitsuno, Y., H. Yoshida, S. Maeda, K. Ogura, Y. Hirata, T. Kawabe, Y. Shiratori, and M. Omata. 2001. Helicobacter pylori induced transactivation of SRE and AP-1 through the ERK signalling pathway in gastric cancer cells. Gut 49:18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moss, S. F., J. Calam, B. Agarwal, S. Wang, and P. R. Holt. 1996. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut 38:498-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moss, S. F., E. M. Sordillo, A. M. Abdalla, V. Makarov, Z. Hanzely, G. I. Perez-Perez, M. J. Blaser, and P. R. Holt. 2001. Increased gastric epithelial cell apoptosis associated with colonization with cagA+ Helicobacter pylori strains. Cancer Res. 61:1406-1411. [PubMed] [Google Scholar]
- 28.Nardone, G., S. Staibano, A. Rocco, E. Mezza, F. P. D'armiento, L. Insabato, A. Coppola, G. Salvatore, A. Lucariello, N. Figura, G. De Rosa, and G. Budillon. 1999. Effect of Helicobacter pylori infection and its eradication on cell proliferation, DNA status, and oncogene expression in patients with chronic gastritis. Gut 44:789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NIH Consensus Development Panel. 1994. Helicobacter pylori in peptic ulcer disease. JAMA 272:65-69. [PubMed] [Google Scholar]
- 30.Ono, K., and J. Han. 2000. The p38 signal transduction pathway: activation and function. Cell. Signal. 12:1-13. [DOI] [PubMed] [Google Scholar]
- 31.Pages, G., P. Lenormand, G. L'Allemain, J. C. Chambard, S. Meloche, and J. Pouyssegur. 1993. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc. Natl. Acad. Sci. USA 90:8319-8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pardo, O. E., A. Arcaro, G. Salerno, S. Raguz, J. Downward, and M. J. Seckl. 2002. Fibroblast growth factor-2 induces translational regulation of Bcl-XL and Bcl-2 via a MEK-dependent pathway: correlation with resistance to etoposide-induced apoptosis. J. Biol. Chem. 277:12040-12046. [DOI] [PubMed] [Google Scholar]
- 33.Parsonnet, J., G. D. Friedman, N. Orentreich, and H. Vogelman. 1997. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 40:297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peek, R. M., Jr., S. F. Moss, K. T. Tham, G. I. Perez-Perez, S. Wang, G. G. Miller, J. C. Atherton, P. R. Holt, and M. J. Blaser. 1997. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J. Natl. Cancer Inst. 89:863-868. [DOI] [PubMed] [Google Scholar]
- 35.Peek, R. M., Jr., M. J. Blaser, D. J. Mays, M. H. Forsyth, T. L. Cover, S. Y. Song, U. Krishna, and J. A. Pietenpol. 1999. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Res. 59:6124-6131. [PubMed] [Google Scholar]
- 36.Rice, P. L., R. J. Goldberg, E. C. Ray, L. J. Driggers, and D. J. Ahnen. 2001. Inhibition of extracellular signal-regulated kinase 1/2 phosphorylation and induction of apoptosis by sulindac metabolites. Cancer Res. 61:1541-1547. [PubMed] [Google Scholar]
- 37.Rokkas, T., S. Ladas, C. Liatsos, E. Petridou, G. Papatheodorou, S. Theocharis, A. Karameris, and S. Raptis. 1999. Relationship of Helicobacter pylori CagA status to gastric cell proliferation and apoptosis. Dig. Dis. Sci. 44:487-493. [DOI] [PubMed] [Google Scholar]
- 38.Rudi, J., D. Kuck, S. Strand, A. von Herbay, S. M. Mariani, P. H. Krammer, P. R. Galle, and W. Stremmel. 1998. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J. Clin. Investig. 102:1506-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwenger, P., P. Bellosta, I. Vietor, C. Basilico, E. Y. Skolnik, and J. Vilcek. 1997. Sodium salicylate induces apoptosis via p38 mitogen-activated protein kinase but inhibits tumor necrosis factor-induced c-Jun N-terminal kinase/stress-activated protein kinase activation. Proc. Natl. Acad. Sci. USA 94:2869-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shakibaei, M., G. Schulze-Tanzil, P. de Souza, T. John, M. Rahmanzadeh, R. Rahmanzadeh, and H. J. Merker. 2001. Inhibition of mitogen-activated protein kinase kinase induces apoptosis of human chondrocytes. J. Biol. Chem. 276:13289-13294. [DOI] [PubMed] [Google Scholar]
- 41.Sharma, S. A., M. K. Tummuru, M. J. Blaser, and L. D. Kerr. 1998. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J. Immunol. 160:2401-2407. [PubMed] [Google Scholar]
- 42.Shirin, H., E. M. Sordillo, S. H. Oh, H. Yamamoto, T. Delohery, I. B. Weinstein, and S. F. Moss. 1999. Helicobacter pylori inhibits the G1 to S transition in AGS gastric epithelial cells. Cancer Res. 59:2277-2281. [PubMed] [Google Scholar]
- 43.Tran, S. E., T. H. Holmstrom, M. Ahonen, V. M. Kahari, and J. E. Eriksson. 2001. MAPK/ERK overrides the apoptotic signaling from Fas, TNF, and TRAIL receptors. J. Biol. Chem. 276:16484-16490. [DOI] [PubMed] [Google Scholar]
- 44.Wagner, S., W. Beil, J. Westermann, R. P. Logan, C. T. Bock, C. Trautwein, J. S. Bleck, and M. P. Manns. 1997. Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology 113:1836-1847. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe, T., M. Tada, H. Nagai, S. Sasaki, and M. Nakao. 1998. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology 115:642-648. [DOI] [PubMed] [Google Scholar]
- 46.Weel, J. F., R. W. van der Hulst, Y. Gerrits, P. Roorda, M. Feller, J. Dankert, G. N. Tytgat, and A. van der Ende. 1996. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J. Infect. Dis. 173:1171-1175. [DOI] [PubMed] [Google Scholar]
- 47.Wessler, S., M. Hocker, W. Fischer, T. C. Wang, S. Rosewicz, R. Haas, B. Wiedenmann, T. F. Meyer, and M. Naumann. 2000. Helicobacter pylori activates the histidine decarboxylase promoter through a mitogen-activated protein kinase pathway independent of pathogenicity island-encoded virulence factors. J. Biol. Chem. 275:3629-3636. [DOI] [PubMed] [Google Scholar]
- 48.Xia, H. H., and N. J. Talley. 2001. Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis. Am. J. Gastroenterol. 96:16-26. [DOI] [PubMed] [Google Scholar]
- 49.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAPKs on apoptosis. Science 270:1326-1331. [DOI] [PubMed] [Google Scholar]