Abstract
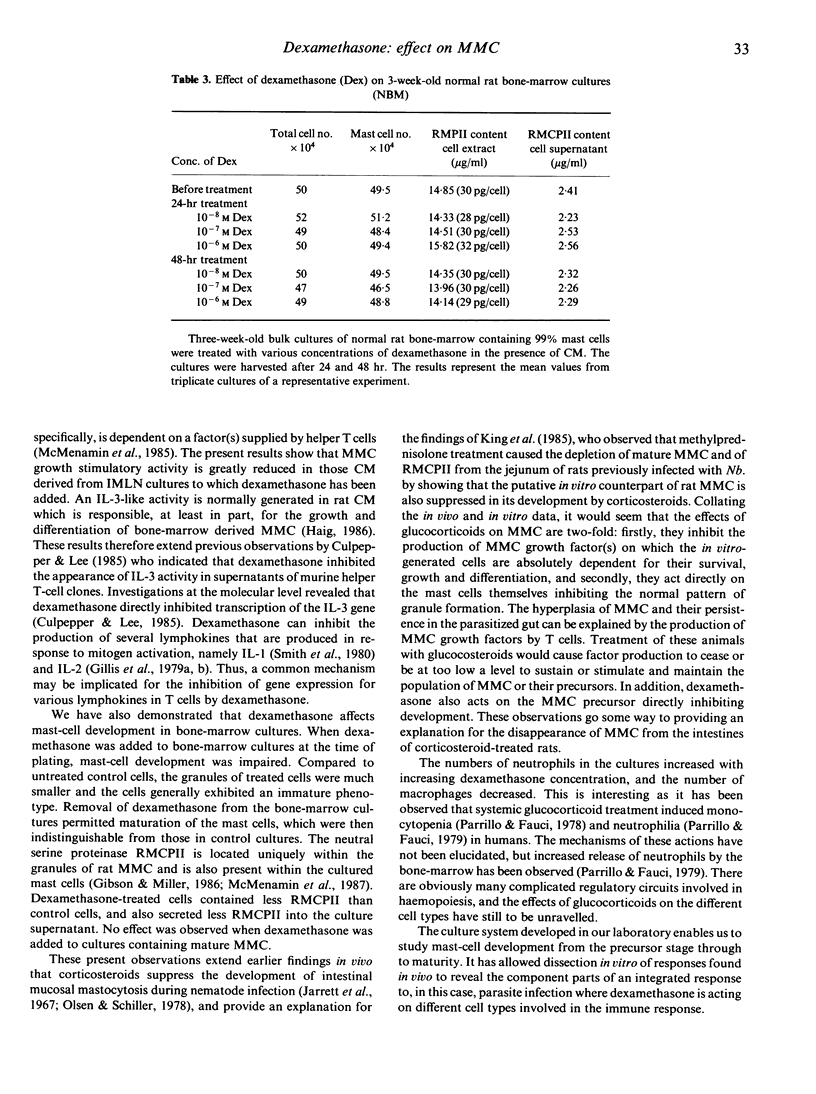
An in vitro culture system was used to investigate the effects of dexamethasone on the production of mucosal mast cell (MMC) growth activity from T cells, and the proliferation and maturation of MMC in culture. The addition of dexamethasone (Dex) to cultures of lymphocytes from Nippostrongylus brasiliensis (Nb.)-infected rats suppressed production of MMC growth activity, as assessed by the lack of MMC growth and differentiation when supernatants of the treated lymphocyte cultures were added to normal rat bone-marrow cultures. Dexamethasone treatment of normal rat bone-marrow cultures affected the maturation of the bone-marrow derived MMC by preventing normal granule development. The ratio of neutrophils:macrophages present in the cultures was also altered. Dexamethasone did not have any detectable effect on mature MMC in culture.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Culpepper J. A., Lee F. Regulation of IL 3 expression by glucocorticoids in cloned murine T lymphocytes. J Immunol. 1985 Nov;135(5):3191–3197. [PubMed] [Google Scholar]
- Gibson S., Miller H. R. Mast cell subsets in the rat distinguished immunohistochemically by their content of serine proteinases. Immunology. 1986 May;58(1):101–104. [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Crabtree G. R., Smith K. A. Glucocorticoid-induced inhibition of T cell growth factor production. I. The effect on mitogen-induced lymphocyte proliferation. J Immunol. 1979 Oct;123(4):1624–1631. [PubMed] [Google Scholar]
- Gillis S., Crabtree G. R., Smith K. A. Glucocorticoid-induced inhibition of T cell growth factor production. II. The effect on the in vitro generation of cytolytic T cells. J Immunol. 1979 Oct;123(4):1632–1638. [PubMed] [Google Scholar]
- Haig D. M., McKee T. A., Jarrett E. E., Woodbury R., Miller H. R. Generation of mucosal mast cells is stimulated in vitro by factors derived from T cells of helminth-infected rats. Nature. 1982 Nov 11;300(5888):188–190. doi: 10.1038/300188a0. [DOI] [PubMed] [Google Scholar]
- Haig D. M., McMenamin C., Gunneberg C., Woodbury R., Jarrett E. E. Stimulation of mucosal mast cell growth in normal and nude rat bone marrow cultures. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4499–4503. doi: 10.1073/pnas.80.14.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscove N. N., Melchers F. Complete replacement of serum by albumin, transferrin, and soybean lipid in cultures of lipopolysaccharide-reactive B lymphocytes. J Exp Med. 1978 Mar 1;147(3):923–933. doi: 10.1084/jem.147.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A., Munck A. Glucocorticoid inhibition of lymphokine secretion by alloreactive T lymphocyte clones. J Immunol. 1984 Aug;133(2):784–791. [PubMed] [Google Scholar]
- King S. J., Miller H. R., Newlands G. F., Woodbury R. G. Depletion of mucosal mast cell protease by corticosteroids: effect on intestinal anaphylaxis in the rat. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1214–1218. doi: 10.1073/pnas.82.4.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G. The nature of the thymus dependency of mucosal mast cells. II. The effect of thymectomy and of depleting recirculating lymphocytes on the response to Nippostrongylus brasilliensis. Cell Immunol. 1979 Oct;47(2):312–322. doi: 10.1016/0008-8749(79)90341-1. [DOI] [PubMed] [Google Scholar]
- McMenamin C., Haig D. M., Gibson S., Newlands G. F., Miller H. R. Phenotypic analysis of mast cell granule proteinases in normal rat bone marrow cultures. Immunology. 1987 Jan;60(1):147–149. [PMC free article] [PubMed] [Google Scholar]
- McMenamin C., Jarrett E. E., Sanderson A. Surface phenotype of T cells producing growth of mucosal mast cells in normal rat bone marrow culture. Immunology. 1985 Jul;55(3):399–403. [PMC free article] [PubMed] [Google Scholar]
- Miller H. R., Jarrett W. F. Immune reactions in mucous membranes. I. Intestinal mast cell response during helminth expulsion in the rat. Immunology. 1971 Mar;20(3):277–288. [PMC free article] [PubMed] [Google Scholar]
- Nawa Y., Miller H. R. Adoptive transfer of the intestinal mast cell response in rats infected with Nippostrongylus brasiliensis. Cell Immunol. 1979 Feb;42(2):225–239. doi: 10.1016/0008-8749(79)90188-6. [DOI] [PubMed] [Google Scholar]
- Olson C. E., Schiller E. L. Strongyloides ratti infections in rats. II. Effects of cortisone treatment. Am J Trop Med Hyg. 1978 May;27(3):527–531. doi: 10.4269/ajtmh.1978.27.527. [DOI] [PubMed] [Google Scholar]
- Parrillo J. E., Fauci A. S. Mechanisms of corticosteroid action on lymphocyte subpopulations. III. Differential effects of dexamethasone administration on subpopulations of effector cells mediating cellular cytotoxicity in man. Clin Exp Immunol. 1978 Jan;31(1):116–125. [PMC free article] [PubMed] [Google Scholar]
- Parrillo J. E., Fauci A. S. Mechanisms of glucocorticoid action on immune processes. Annu Rev Pharmacol Toxicol. 1979;19:179–201. doi: 10.1146/annurev.pa.19.040179.001143. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Miller H. R. Quantitative analysis of mucosal mast cell protease in the intestines of Nippostrongylus-infected rats. Immunology. 1982 Jul;46(3):487–495. [PMC free article] [PubMed] [Google Scholar]