Abstract
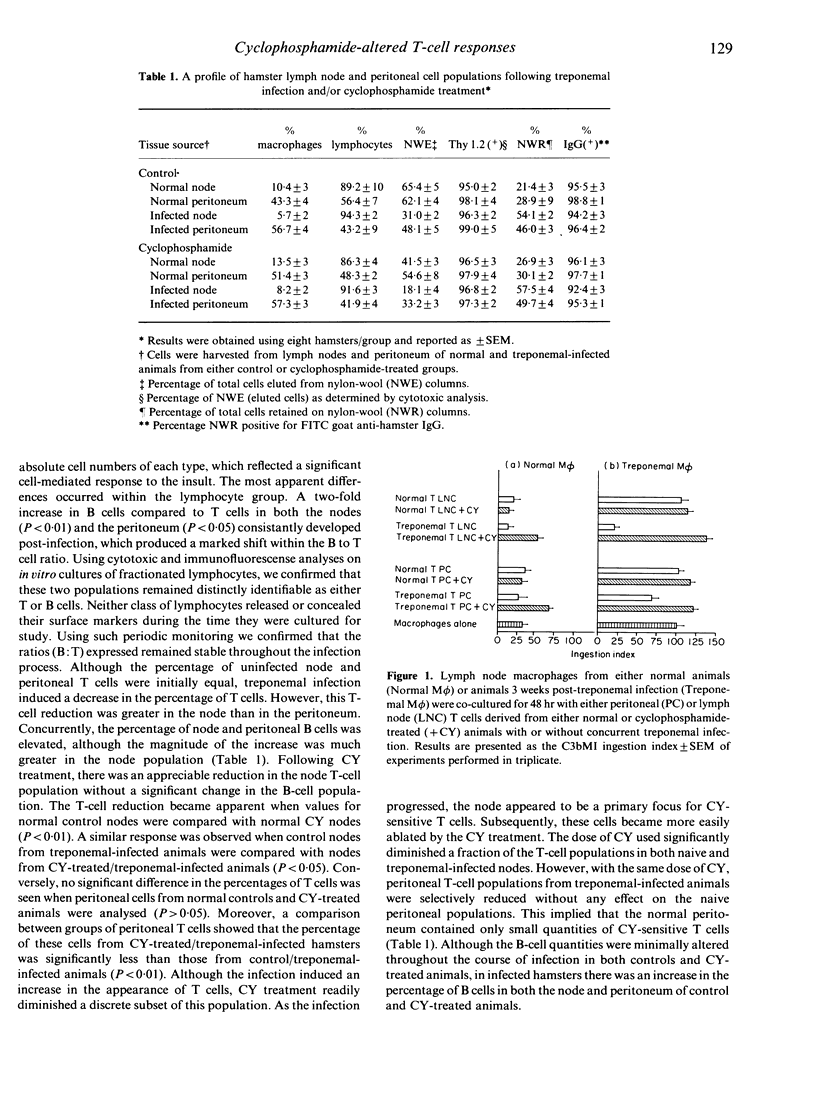
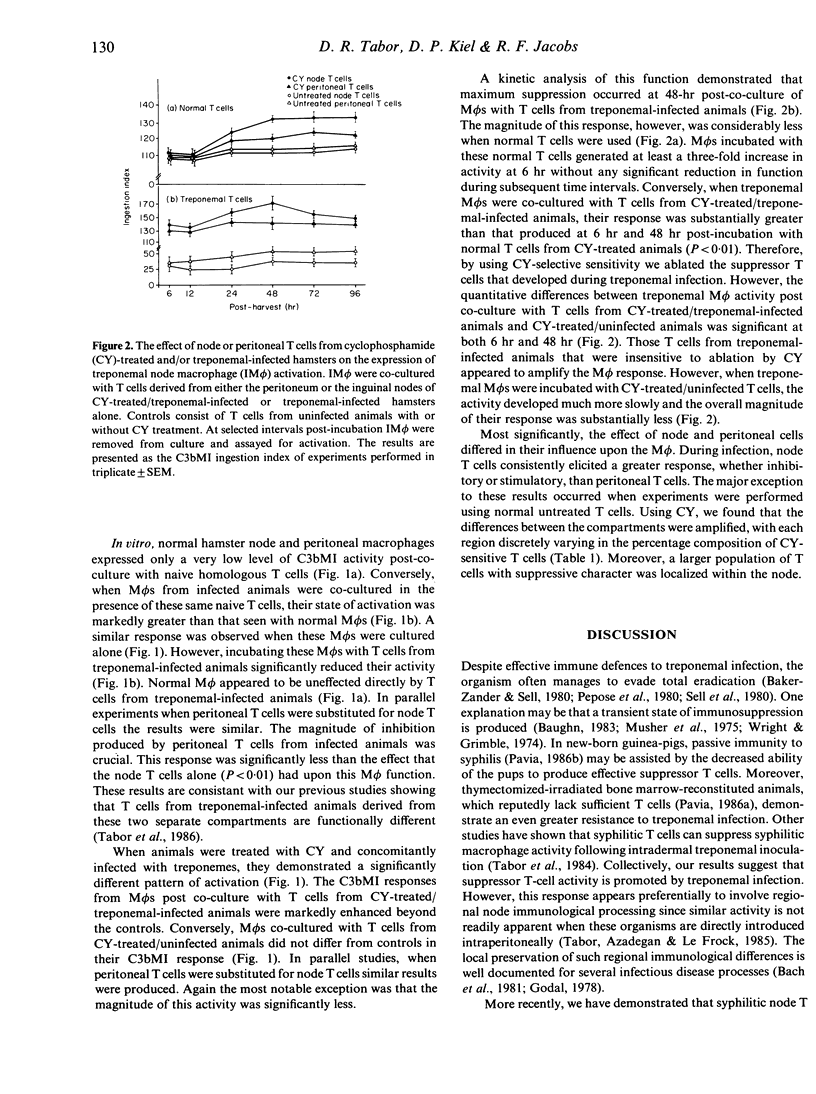
When hamsters were infected with Treponema pallidum subspecies endemicum, the composition and activity of the cellular immune components were markedly altered compared to those of sham-infected controls. A population of suppressor T cells (Ts) developed that diminished the ability of the macrophage (M phi) to perform C3b receptor-mediated ingestion (C3bMI) of erythrocytes coated with antibody and complement. Using cyclophosphamide (CY) we examined node and peritoneal cells to determine their role in regulating M phi activity during this infection. In vitro the node and peritoneal T cells from treponemal-infected/CY-treated animals showed considerably less suppressive activity than treponemal-infected/untreated T cells when co-cultured with M phi from infected animals. This response was greater with node T cells compared to peritoneal T cells. Moreover, a quantitative analysis of the mononuclear leucocyte populations from each of these regions showed that CY-treated/uninfected animals had a decreased percentage of node T cells. Despite this reduction of node T cells, peritoneal T-cell populations were only minimally reduced. However, treponemal-infected hamsters concomitantly treated with CY had a significant reduction in the T-cell percentages in both compartments. These results imply that, during this infection, most Ts generated in the node remain there although some are dispersed to supplementary regions. Thus, the development of a suppressor system that effects M phi function may be one way in which treponemes escape total elimination by the host.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. A., Chatenoud L., Wallach D., Phan Dinh Tuy F., Cottenot F. Studies on T cell subsets and functions in leprosy. Clin Exp Immunol. 1981 Jun;44(3):491–500. [PMC free article] [PubMed] [Google Scholar]
- Bagasra O., Damjanov I. Ability of macrophages to process and present Treponema pallidum Bosnia A strain antigens in experimental syphilis of syrian hamsters. Infect Immun. 1982 Apr;36(1):176–183. doi: 10.1128/iai.36.1.176-183.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O., Tabor D. Irradiation- and cyclophosphamide-induced alterations in Syrian hamster T-cell population activity. J Leukoc Biol. 1986 Feb;39(2):183–192. doi: 10.1002/jlb.39.2.183. [DOI] [PubMed] [Google Scholar]
- Baker-Zander S., Sell S. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Demonstration of long-lasting cellular immunity. Am J Pathol. 1980 Nov;101(2):387–414. [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Tung K. S., Musher D. M. Detection of circulating immune complexes in the sera of rabbits with experimental syphilis: possible role in immunoregulation. Infect Immun. 1980 Aug;29(2):575–582. doi: 10.1128/iai.29.2.575-582.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. I. The demonstration of resistance conferred by passive immunization. J Immunol. 1976 Jul;117(1):191–196. [PubMed] [Google Scholar]
- Blanco D. R., Miller J. N., Hanff P. A. Humoral immunity in experimental syphilis: the demonstration of IgG as a treponemicidal factor in immune rabbit serum. J Immunol. 1984 Nov;133(5):2693–2697. [PubMed] [Google Scholar]
- Godal T. Immunological aspects of leprosy--present status. Prog Allergy. 1978;25:211–242. [PubMed] [Google Scholar]
- HOLLANDER D. H., TURNER T. B. The role of temperature in experimental treponemal infection. Am J Syph Gonorrhea Vener Dis. 1954 Nov;38(6):489–505. [PubMed] [Google Scholar]
- Hanff P. A., Fernandez C., Folds J. D. Percoll-purified Treponema pallidum, an improved fluorescent treponemal antibody-absorbed antigen. J Clin Microbiol. 1986 May;23(5):980–982. doi: 10.1128/jcm.23.5.980-982.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff P. A., Norris S. J., Lovett M. A., Miller J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984 Oct-Dec;11(4):275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A. Activation of macrophages by products of lymphocytes from normal and syphilitic rabbits. Infect Immun. 1982 Jul;37(1):64–69. doi: 10.1128/iai.37.1.64-69.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukehart S. A., Miller J. N. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978 Nov;121(5):2014–2024. [PubMed] [Google Scholar]
- Maret S. M., Baseman J. B., Folds J. D. Cell-mediated immunity in Treponema pallidum infected rabbits: in vitro response of splenic and lymph node lymphocytes to mitogens and specific antigens. Clin Exp Immunol. 1980 Jan;39(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Jones R. H., Jones A. M. Lymphocyte transformation in syphilis: an in vitro correlate of immune suppression in vivo? Infect Immun. 1975 Jun;11(6):1261–1264. doi: 10.1128/iai.11.6.1261-1264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Baseman J. B., Folds J. D. Selective response of lymphocytes from Treponema pallidum-infected rabbits to mitogens and Treponema reiteri. Infect Immun. 1977 Feb;15(2):417–422. doi: 10.1128/iai.15.2.417-422.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S. Enhanced primary resistance to Treponema pallidum infection and increased susceptibility to toxoplasmosis in T-cell-depleted guinea pigs. Infect Immun. 1986 Aug;53(2):305–311. doi: 10.1128/iai.53.2.305-311.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Depression of lymphocyte response to concanavalin A in rabbits infected with Treponema pallidum (Nichols strain). Infect Immun. 1976 Jul;14(1):320–322. doi: 10.1128/iai.14.1.320-322.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Niederbuhl C. J. Adoptive transfer of anti-syphilis immunity with lymphocytes from Treponema pallidum-infected guinea pigs. J Immunol. 1985 Oct;135(4):2829–2834. [PubMed] [Google Scholar]
- Pavia C. S. Transfer of resistance to syphilitic infection from maternal to newborn guinea pigs. Infect Immun. 1986 Jan;51(1):365–368. doi: 10.1128/iai.51.1.365-368.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepose J. S., Bishop N. H., Feigenbaum S., Miller J. N., Zeltzer P. M. The humoral immune response in rabbits infected with Treponema pallidum: Comparison of antibody levels measured by the staphylococcal protein A-IgG (SPA-TP) microassay with VDRL, FTA-Abs, and TPI antibody responses during the development of acquired resistance to challenge. Sex Transm Dis. 1980 Jul-Sep;7(3):125–129. [PubMed] [Google Scholar]
- Sell S., Gamboa D., Baker-Zander S. A., Lukehart S. A., Miller J. N. Host response to Treponema pallidum in intradermally-infected rabbits: evidence for persistence of infection at local and distant sites. J Invest Dermatol. 1980 Dec;75(6):470–475. doi: 10.1111/1523-1747.ep12524230. [DOI] [PubMed] [Google Scholar]
- Tabor D. R., Azadegan A. A., LeFrock J. L. The participation of activated peritoneal macrophages in Treponema pallidum subspecies pertenue infection in Syrian hamsters. J Leukoc Biol. 1985 Nov;38(5):625–634. doi: 10.1002/jlb.38.5.625. [DOI] [PubMed] [Google Scholar]
- Tabor D. R., Azadegan A. A., Schell R. F., Lefrock J. L. Inhibition of macrophage C3b-mediated ingestion by syphilitic hamster T cell-enriched fractions. J Immunol. 1984 Nov;133(5):2698–2705. [PubMed] [Google Scholar]
- Tabor D. R., Bagasra O., Jacobs R. F. Treponemal infection specifically enhances node T-cell regulation of macrophage activity. Infect Immun. 1986 Oct;54(1):21–27. doi: 10.1128/iai.54.1.21-27.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte P. L., Streilein J. W. Development and ontogeny of hamster T cell subpopulations. J Immunol. 1986 Jul 1;137(1):45–54. [PubMed] [Google Scholar]
- Witte P. L., Streilein J. W. Monoclonal antibodies to hamster class II MHC molecules distinguish T and B cells. J Immunol. 1983 May;130(5):2282–2286. [PubMed] [Google Scholar]
- Witte P. L., Streilein J. W. Thy-1 antigen is present on B and T lymphocytes of the Syrian hamster. J Immunol. 1983 Dec;131(6):2903–2907. [PubMed] [Google Scholar]
- Wright D. J., Grimble A. S. Why is the infectious stage of syphilis prolonged? Br J Vener Dis. 1974 Feb;50(1):45–49. doi: 10.1136/sti.50.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K. E., Clark D. A., Rawls W. E. Differences in lymphocyte responsiveness to lymphokines in two inbred strains of Syrian hamster. J Immunol. 1984 Jul;133(1):286–292. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


