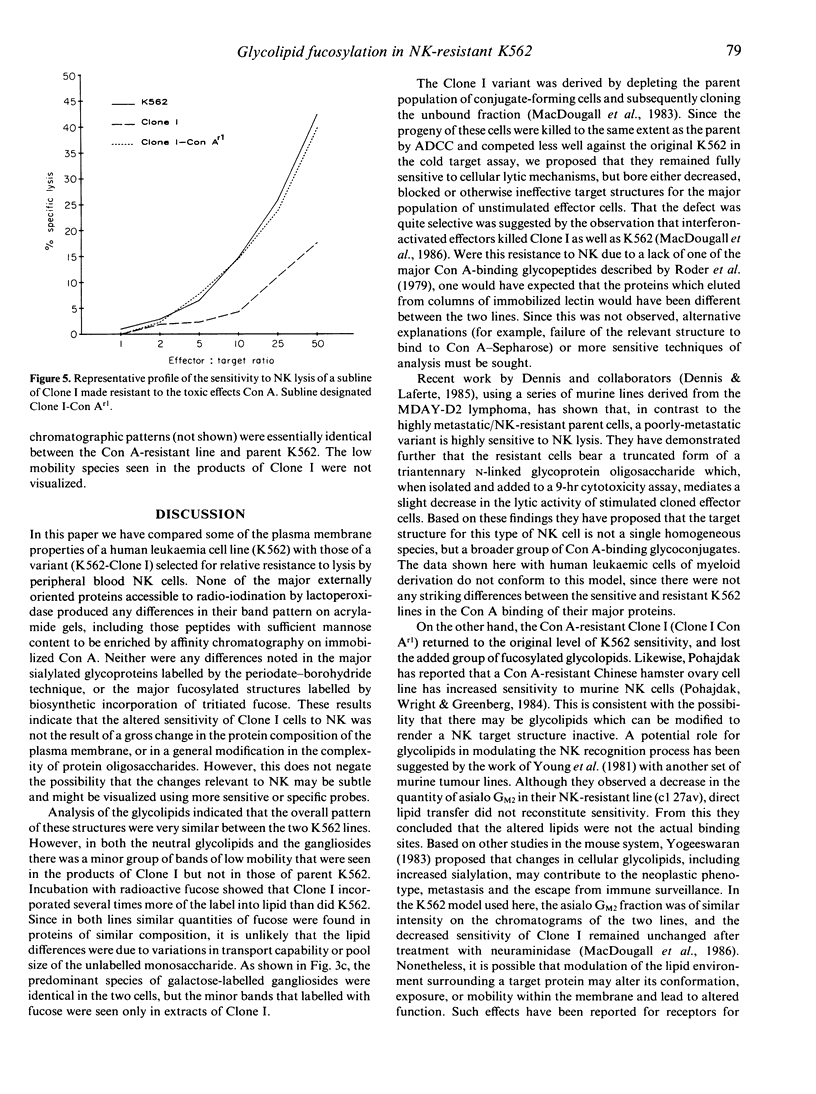
Abstract
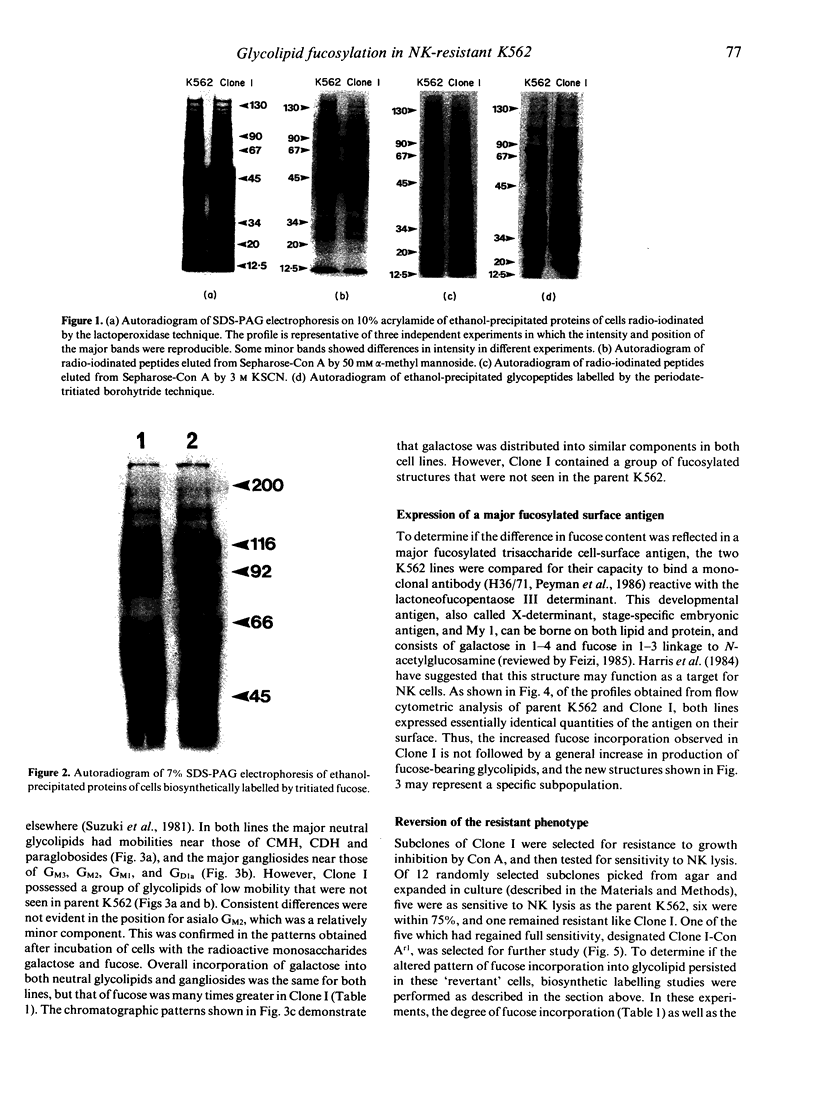
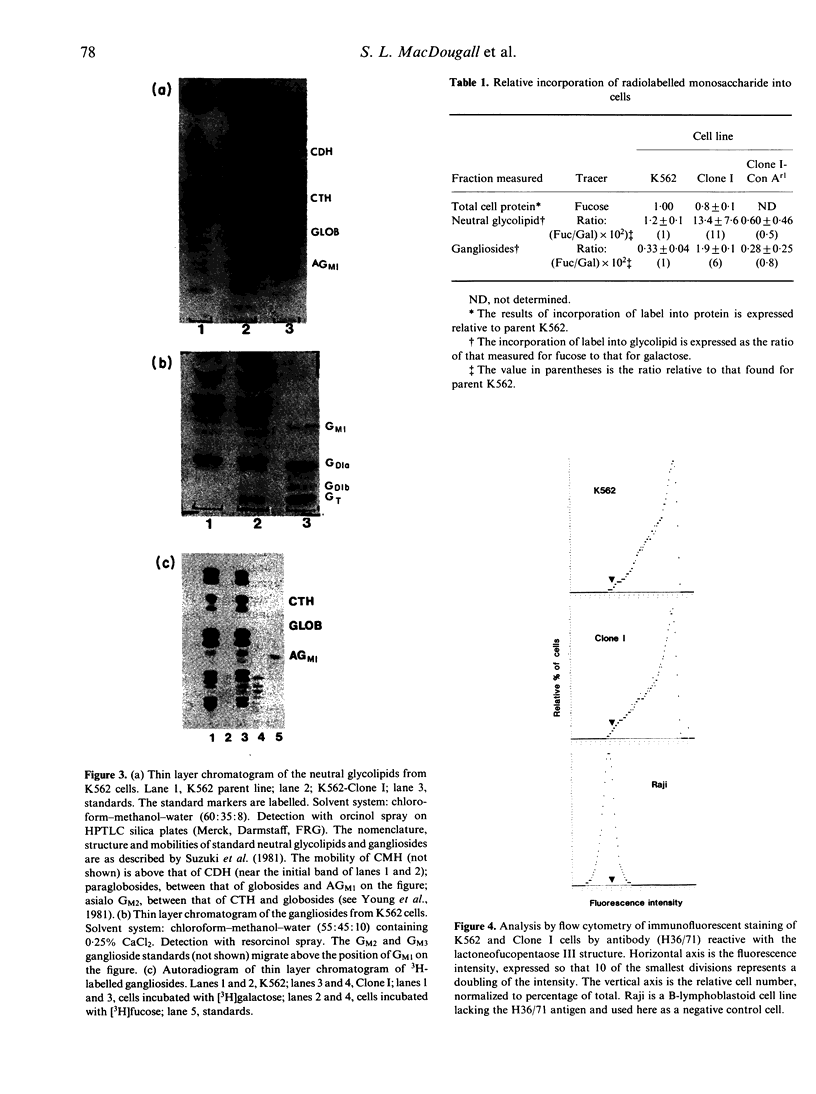
A subpopulation of human lymphoid cells called natural killers is able to lyse certain normal and neoplastic targets in an in vitro cytotoxicity assay. The molecules which enable them to recognize sensitive cells, or permit tumour cells to escape remain unknown. In the studies described here we have compared some of the plasma membrane characteristics of a NK-sensitive human leukaemia cell line (K562) with those of a partially resistant subclone derived from it (K562-Clone I). Gel electrophoresis of cell-surface proteins radiolabelled by lactoperoxidase-catalysed iodination, periodate-borohydride tritiation, or biosynthetically by incubation with [3H]fucose did not reveal any reproducible differences between the sensitive and resistant lines. However, analysis of glycolipids showed that Clone I incorporated significantly more fucose than did the parental line, and that it synthesized a minor population of complex structures not found in the original K562. A subclone of Clone I (Clone I-Con Ar1), made resistant to the toxic effects of concanavalin A, became sensitive once again to NK, and showed the parental glycolipid profile. These results suggest that the Clone I line, selected for resistance to NK, may have altered one or more of its intermediate oligosaccharides or pathways of fucose incorporation into glycolipid, and points to one process by which a tumor cell might modulate its surface to escape recognition by natural killers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines M. G., Lafleur F. L., Holbein B. E. Involvement of transferrin and transferrin receptors in human natural killer effector:target interaction. Immunol Lett. 1983;7(1):51–55. doi: 10.1016/0165-2478(83)90055-x. [DOI] [PubMed] [Google Scholar]
- Bremer E. G., Hakomori S., Bowen-Pope D. F., Raines E., Ross R. Ganglioside-mediated modulation of cell growth, growth factor binding, and receptor phosphorylation. J Biol Chem. 1984 Jun 10;259(11):6818–6825. [PubMed] [Google Scholar]
- Dennis J. W., Laferté S. Recognition of asparagine-linked oligosaccharides on murine tumor cells by natural killer cells. Cancer Res. 1985 Dec;45(12 Pt 1):6034–6040. [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977 Aug 25;252(16):5888–5894. [PubMed] [Google Scholar]
- Harris J. F., Chin J., Jewett M. A., Kennedy M., Gorczynski R. M. Monoclonal antibodies against SSEA-1 antigen: binding properties and inhibition of human natural killer cell activity against target cells bearing SSEA-1 antigen. J Immunol. 1984 May;132(5):2502–2509. [PubMed] [Google Scholar]
- Hiserodt J. C., Britvan L. J., Targan S. R. Characterization of the cytolytic reaction mechanism of the human natural killer (NK) lymphocyte: resolution into binding, programming, and killer cell-independent steps. J Immunol. 1982 Oct;129(4):1782–1787. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacDougall S. L., Shustik C., Sullivan A. K. Target cell specificity of human natural killer (NK) cells. I. Development of an NK-resistant subline of K562. Cell Immunol. 1983 Feb 15;76(1):39–48. doi: 10.1016/0008-8749(83)90346-5. [DOI] [PubMed] [Google Scholar]
- MacDougall S. L., Shustik C., Sullivan A. K. Target cell specificity of human natural killer cells. II. Apparent change with activation. Cell Immunol. 1986 Dec;103(2):352–364. doi: 10.1016/0008-8749(86)90095-x. [DOI] [PubMed] [Google Scholar]
- Muller C., Shinitzky M. Modulation of transferrin receptors in bone marrow cells by changes in lipid fluidity. Br J Haematol. 1979 Jul;42(3):355–362. doi: 10.1111/j.1365-2141.1979.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Peyman J. A., Schwarting G. A., Sullivan A. K. Differences in the plasma membrane glycoproteins of cultured myeloblastoid and promyelocytic human leukemia (HL60) cells. Leuk Res. 1986;10(8):973–988. doi: 10.1016/0145-2126(86)90250-x. [DOI] [PubMed] [Google Scholar]
- Pohajdak B., Wright J. A., Greenberg A. J. An oligosaccharide biosynthetic defect in concanavalin A-resistant Chinese hamster ovary (CHO) cells that enhances NK reactivity in vitro and in vivo. J Immunol. 1984 Nov;133(5):2423–2429. [PubMed] [Google Scholar]
- Pross H. F., Luk S. S., Baines M. G. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. V. The role of serum-derived heterologous membrane antigens. Int J Cancer. 1978 Mar 15;21(3):291–298. doi: 10.1002/ijc.2910210307. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Rosén A., Fenyö E. M., Troy F. A. Target-effector interaction in the natural killer cell system: isolation of target structures. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1405–1409. doi: 10.1073/pnas.76.3.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting G. A., Summers A. Gangliotetraosylceramide is a T cell differentiation antigen associated with natural cell-mediated cytotoxicity. J Immunol. 1980 Apr;124(4):1691–1694. [PubMed] [Google Scholar]
- Suzuki A., Karol R. A., Kundu S. K., Marcus D. M. Glycosphingolipids of K562 cells: a chemical and immunological analysis. Int J Cancer. 1981 Sep 15;28(3):271–276. doi: 10.1002/ijc.2910280304. [DOI] [PubMed] [Google Scholar]
- Vodinelich L., Sutherland R., Schneider C., Newman R., Greaves M. Receptor for transferrin may be a "target" structure for natural killer cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):835–839. doi: 10.1073/pnas.80.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh F. S., Crumpton M. J. Orientation of cell-surface antigens in the lipid bilayer of lymphocyte plasma membrane. Nature. 1977 Sep 22;269(5626):307–311. doi: 10.1038/269307a0. [DOI] [PubMed] [Google Scholar]
- Williams M. A., McCluer R. H. The use of Sep-Pak C18 cartridges during the isolation of gangliosides. J Neurochem. 1980 Jul;35(1):266–269. doi: 10.1111/j.1471-4159.1980.tb12515.x. [DOI] [PubMed] [Google Scholar]
- Yogeeswaran G. Cell surface glycolipids and glycoproteins in malignant transformation. Adv Cancer Res. 1983;38:289–350. doi: 10.1016/s0065-230x(08)60191-8. [DOI] [PubMed] [Google Scholar]
- Yogeeswaran G., Gronberg A., Hansson M., Dalianis T., Kiessling R., Welsh R. M. Correlation of glycosphingolipids and sialic acid in YAC-1 lymphoma variants with their sensitivity to natural killer-cell-mediated lysis. Int J Cancer. 1981 Oct 15;28(4):517–526. doi: 10.1002/ijc.2910280419. [DOI] [PubMed] [Google Scholar]
- Young W. W., Jr, Durdik J. M., Urdal D., Hakomori S., Henney C. S. Glycolipid expression in lymphoma cell variants: chemical quantity, immunologic reactivity, and correlations with susceptibility to NK cells. J Immunol. 1981 Jan;126(1):1–6. [PubMed] [Google Scholar]