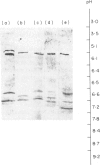
Abstract
Mice were immunized with various antigens in complete Freund's adjuvant following various injection schedules. Hybridomas were produced from the spleens of these immunized mice and examined for production of antibodies directed against the antigen injected and against a panel of self (tubulin, actin, myosin, DNA) and non-self antigens (myoglobin, spectrin, peroxidase, trinitrobenzene). Two to five percent of the hybrids were found to secrete polyspecific antibodies able to react with two or more antigens of the panel. Several of these hybrids were subcloned and expanded into ascites. The monoclonal immunoglobulins they secreted were isolated and shown to be IgM (kappa) and to possess the polyspecific antibody function. Several hybrids were also found to secrete antibodies reacting with the immunizing antigen as well as one or more antigens of the panel. The antibody secreted by one subclone which reacts with both the immunizing antigen, prolactin and one of the panel antigens, TNP, has been isolated using a DNP-immunoadsorbent. The isolated antibody was found to be a monoclonal IgM (kappa) immunoglobulin and to react both with prolactin and TNP. The hypothesis is advanced that cells carrying polyspecific natural antibodies as receptors after a given antigenic stimulation proliferate into cells producing highly specific antibodies for epitopes of that given antigen; the cells with polyspecific receptors will be continuously replaced by new cells probably on bone-marrow origin.
Full text
PDF


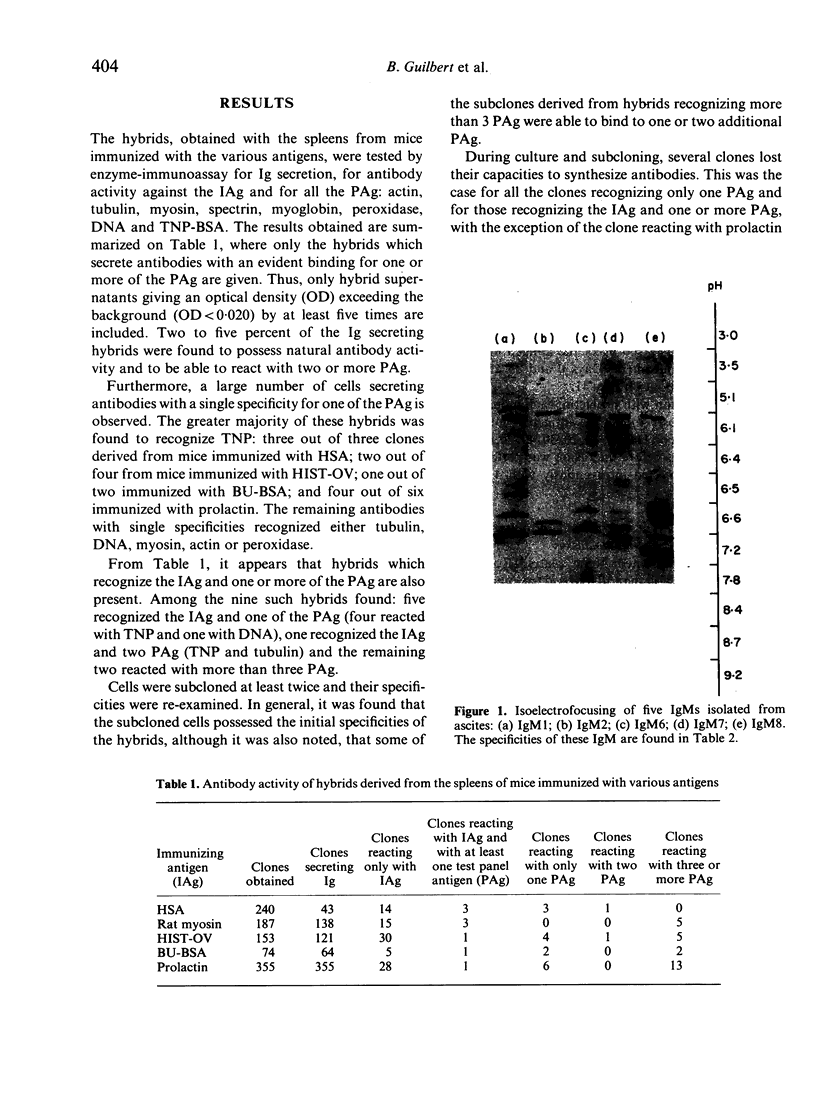
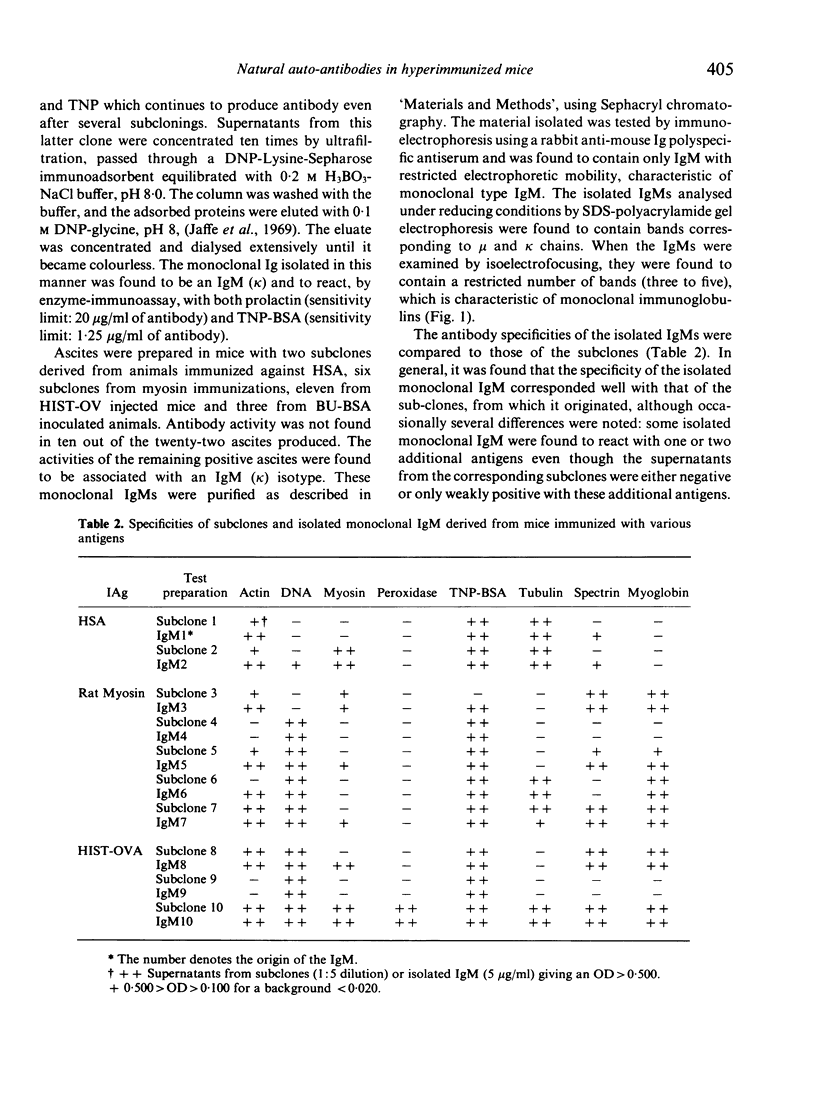
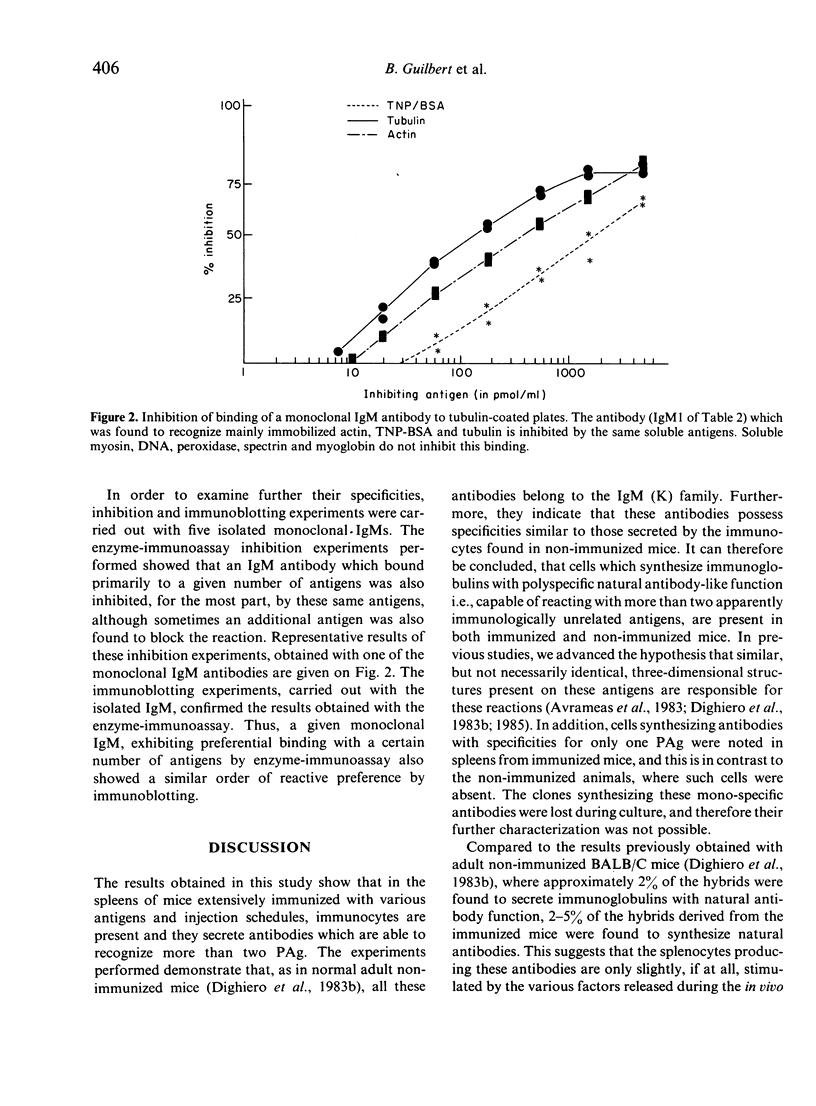


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Dighiero G., Lymberi P., Guilbert B. Studies on natural antibodies and autoantibodies. Ann Immunol (Paris) 1983 Jul-Aug;134D(1):103–113. [PubMed] [Google Scholar]
- Avrameas S., Guilbert B., Dighiero G. Natural antibodies against tubulin, actin myoglobin, thyroglobulin, fetuin, albumin and transferrin are present in normal human sera, and monoclonal immunoglobulins from multiple myeloma and Waldenström's macroglobulinemia may express similar antibody specificities. Ann Immunol (Paris) 1981 Mar-Apr;132C(2):231–236. doi: 10.1016/0769-2625(81)90031-3. [DOI] [PubMed] [Google Scholar]
- BOYDEN S. AUTOIMMUNITY AND INFLAMMATION. Nature. 1964 Jan 11;201:200–201. doi: 10.1038/201200a0. [DOI] [PubMed] [Google Scholar]
- Bouvet J. P., Pires R., Pillot J. A modified gel filtration technique producing an unusual exclusion volume of IgM: a simple way of preparing monoclonal IgM. J Immunol Methods. 1984 Feb 10;66(2):299–305. doi: 10.1016/0022-1759(84)90341-7. [DOI] [PubMed] [Google Scholar]
- Cairns E., Block J., Bell D. A. Anti-DNA autoantibody-producing hybridomas of normal human lymphoid cell origin. J Clin Invest. 1984 Sep;74(3):880–887. doi: 10.1172/JCI111505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J. Large numbers of cells in normal mice produce antibody components of isologous erythrocytes. Nature. 1974 Dec 20;252(5485):749–751. doi: 10.1038/252749a0. [DOI] [PubMed] [Google Scholar]
- Dighiero G., Guilbert B., Avrameas S. Naturally occurring antibodies against nine common antigens in humans sera. II. High incidence of monoclonal Ig exhibiting antibody activity against actin and tubulin and sharing antibody specificities with natural antibodies. J Immunol. 1982 Jun;128(6):2788–2792. [PubMed] [Google Scholar]
- Dighiero G., Guilbert B., Fermand J. P., Lymberi P., Danon F., Avrameas S. Thirty-six human monoclonal immunoglobulins with antibody activity against cytoskeleton proteins, thyroglobulin, and native DNA: immunologic studies and clinical correlations. Blood. 1983 Aug;62(2):264–270. [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Holmberg D., Lundquist I., Coutinho A., Avrameas S. High frequency of natural autoantibodies in normal newborn mice. J Immunol. 1985 Feb;134(2):765–771. [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Mazié J. C., Rouyre S., Butler-Browne G. S., Whalen R. G., Avrameas S. Murine hybridomas secreting natural monoclonal antibodies reacting with self antigens. J Immunol. 1983 Nov;131(5):2267–2272. [PubMed] [Google Scholar]
- ERLANGER B. F., BEISER S. M. ANTIBODIES SPECIFIC FOR RIBONUCLEOSIDES AND RIBONUCLEOTIDES AND THEIR REACTION WITH DNA. Proc Natl Acad Sci U S A. 1964 Jul;52:68–74. doi: 10.1073/pnas.52.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U., Rachmilewitz E. A., Peleg A., Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984 Nov 1;160(5):1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbert B., Dighiero G., Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982 Jun;128(6):2779–2787. [PubMed] [Google Scholar]
- Jaffe B. M., Eisen H. N., Simms E. S., Potter M. Myeloma proteins with anti-hapten antibody activity: epsilon-2,4-dinitrophenyl lysine binding by the protein produced by mouse plasmacytoma MOPC-460. J Immunol. 1969 Oct;103(4):872–874. [PubMed] [Google Scholar]
- Karsenti E., Guilbert B., Bornens M., Avrameas S. Antibodies to tubulin in normal nonimmunized animals. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3997–4001. doi: 10.1073/pnas.74.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. R., Eisen H. N. Preparation and characterization of antibodies specific for the 2,4,6-trinitrophenyl group. Biochemistry. 1966 Nov;5(11):3385–3395. doi: 10.1021/bi00875a001. [DOI] [PubMed] [Google Scholar]
- Longenecker B. M., Mosmann T. R. "Natural" antibodies to chicken MHC antigens are present in mice, rats, humans, alligators and allogeneic chickens. Immunogenetics. 1980;11(3):293–302. doi: 10.1007/BF01567795. [DOI] [PubMed] [Google Scholar]
- Lutz H. U., Flepp R., Stringaro-Wipf G. Naturally occurring autoantibodies to exoplasmic and cryptic regions of band 3 protein, the major integral membrane protein of human red blood cells. J Immunol. 1984 Nov;133(5):2610–2618. [PubMed] [Google Scholar]
- Lutz H. U., Wipf G. Naturally occurring autoantibodies to skeletal proteins from human red blood cells. J Immunol. 1982 Apr;128(4):1695–1699. [PubMed] [Google Scholar]
- Martin W. J., Martin S. E. Thymus reactive IgM autoantibodies in normal mouse sera. Nature. 1975 Apr 24;254(5502):716–718. doi: 10.1038/254716a0. [DOI] [PubMed] [Google Scholar]
- Mead G. M., Cowin P., Whitehouse J. M. Antitubulin antibody in healthy adults and patients with infectious mononucleosis and its relationship to smooth muscle antibody (SMA). Clin Exp Immunol. 1980 Feb;39(2):328–336. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Prabhakar B. S., Saegusa J., Onodera T., Notkins A. L. Lymphocytes capable of making monoclonal autoantibodies that react with multiple organs are a common feature of the normal B cell repertoire. J Immunol. 1984 Dec;133(6):2815–2817. [PubMed] [Google Scholar]
- Steele E. J., Cunningham A. J. High proportion of Ig-producing cells making autoantibody in normal mice. Nature. 1978 Aug 3;274(5670):483–484. doi: 10.1038/274483a0. [DOI] [PubMed] [Google Scholar]
- Ternynck T., Avrameas S. Conjugation of p-benzoquinone treated enzymes with antibodies and Fab fragments. Immunochemistry. 1977 Nov-Dec;14(11-12):767–774. doi: 10.1016/0019-2791(77)90352-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]