Abstract
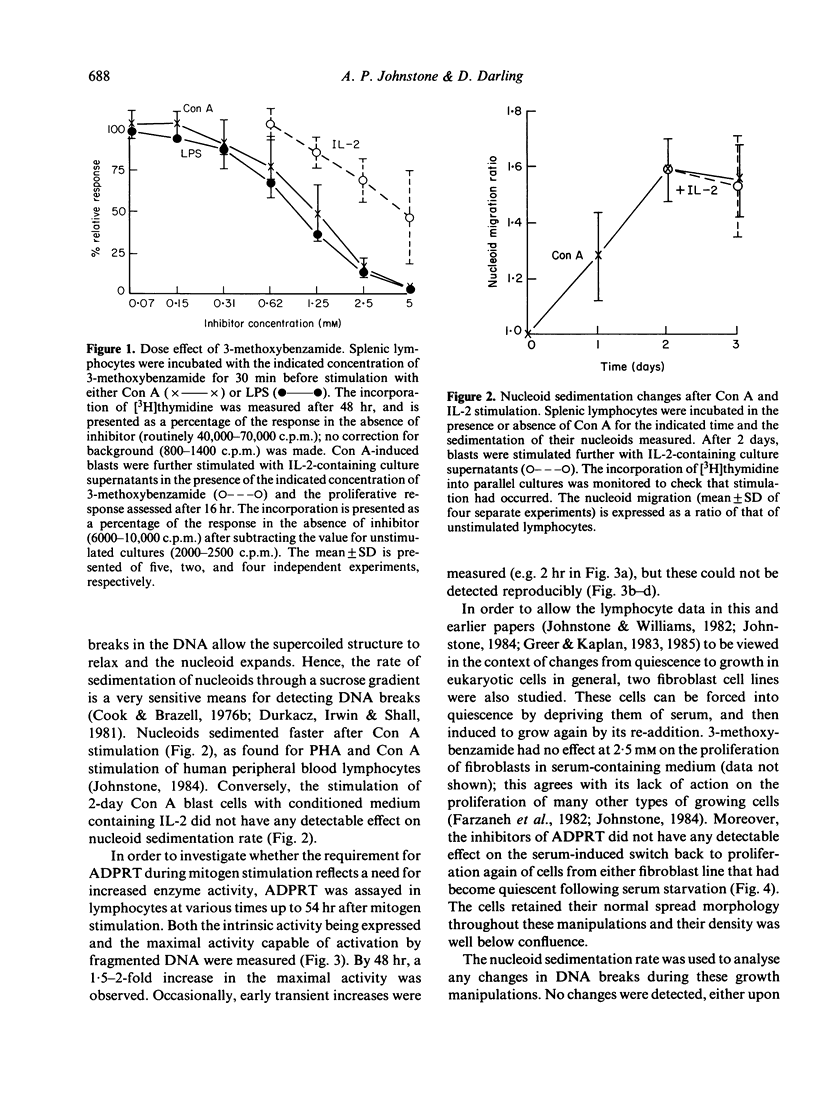
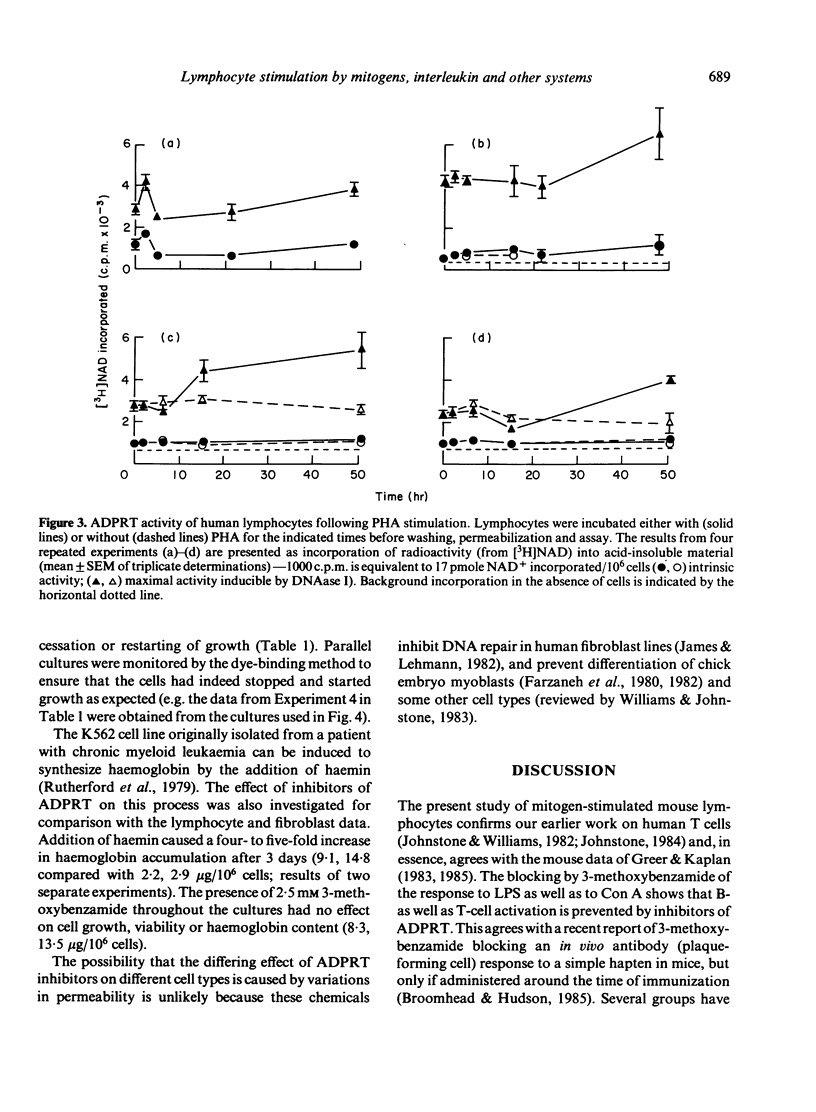
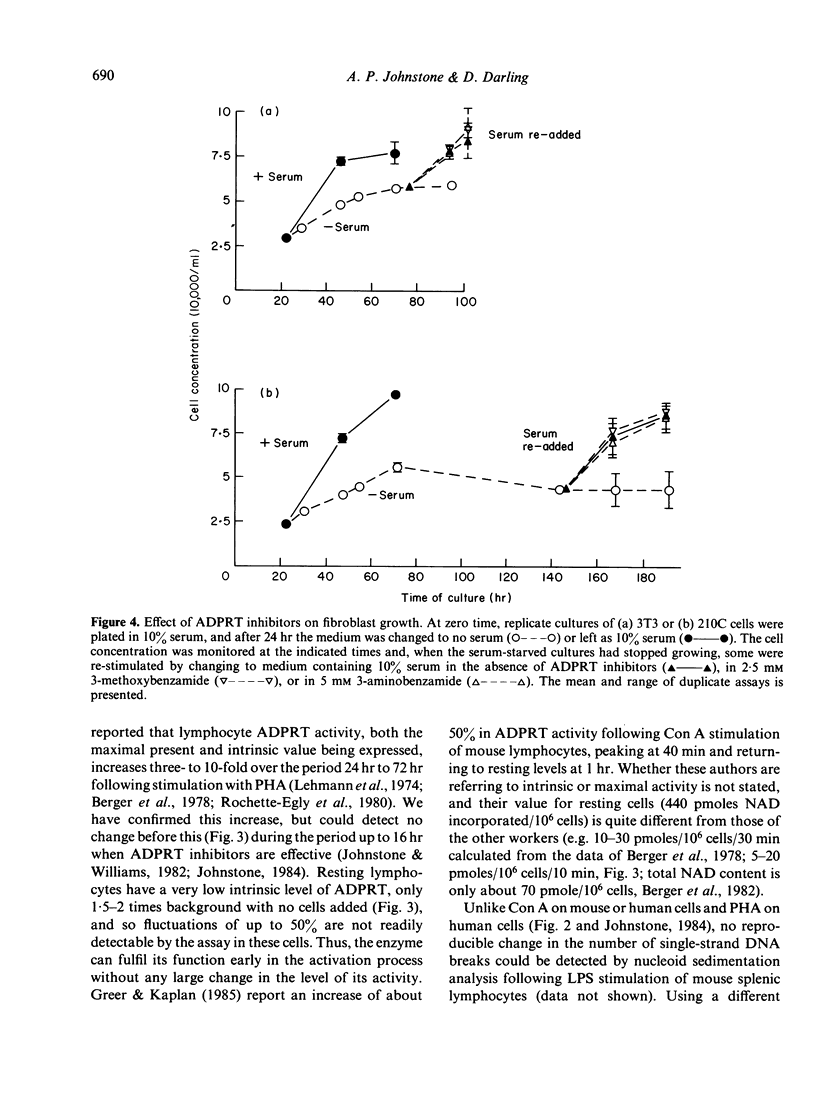
The requirement for activity of the enzyme ADP-ribosyl transferase (ADPRT) and changes in single-strand DNA breaks were assessed during the initial stimulation of quiescent murine splenic lymphocytes with mitogen alone, the stimulation of activated blasts with IL-2-containing medium and, for comparison, the serum stimulation of quiescent fibroblasts and the induction of haemoglobin synthesis in an erythromyeloid cell line K562. Inhibitors of ADPRT, at concentrations previously found to have no effect on the proliferation of lymphoblastoid cell lines, blocked the stimulation of spleen cells by Con A or LPS; non-inhibitory analogues had much less effect. No early increase in ADPRT activity after mitogenic stimulation was detectable. The rejoining of single-strand breaks was observed after stimulation of splenic lymphocytes with Con A, but not consistently with LPS. Conversely, ADPRT inhibitors had only little effect on the IL-2-induced stimulation of Con A blasts, and no effect on the stimulation of fibroblasts or K562. Neither were any changes in strand breaks associated with these systems. These findings implicate ADPRT activity and the rejoining of strand breaks in the early mitogenic response as being distinct from later IL-2 activation and changes from quiescence to growth in other cell types.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger N. A., Adams J. W., Sikorski G. W., Petzold S. J., Shearer W. T. Synthesis of DNA and poly(adenosine diphosphate ribose) in normal and chronic lymphocytic leukemia lymphocytes. J Clin Invest. 1978 Jul;62(1):111–118. doi: 10.1172/JCI109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger N. A., Berger S. J., Sikorski G. W., Catino D. M. Amplification of pyridine nucleotide pools in mitogen-stimulated human lymphocytes. Exp Cell Res. 1982 Jan;137(1):79–88. doi: 10.1016/0014-4827(82)90010-6. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A. Conformational constraints in nuclear DNA. J Cell Sci. 1976 Nov;22(2):287–302. doi: 10.1242/jcs.22.2.287. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A. Detection and repair of single-strand breaks in nuclear DNA. Nature. 1976 Oct 21;263(5579):679–682. doi: 10.1038/263679a0. [DOI] [PubMed] [Google Scholar]
- Dean A., Ley T. J., Humphries R. K., Fordis M., Schechter A. N. Inducible transcription of five globin genes in K562 human leukemia cells. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5515–5519. doi: 10.1073/pnas.80.18.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkacz B. W., Shall S., Irwin J. The effect of inhibition of (ADP-ribose)n biosynthesis on DNA repair assayed by the nucleoid technique. Eur J Biochem. 1981 Dec;121(1):65–69. doi: 10.1111/j.1432-1033.1981.tb06430.x. [DOI] [PubMed] [Google Scholar]
- Farzaneh F., Zalin R., Brill D., Shall S. DNA strand breaks and ADP-ribosyl transferase activation during cell differentiation. Nature. 1982 Nov 25;300(5890):362–366. doi: 10.1038/300362a0. [DOI] [PubMed] [Google Scholar]
- Greer W. L., Kaplan J. G. DNA strand breaks in murine lymphocytes: induction by purine and pyrimidine analogues. Biochem Biophys Res Commun. 1983 Sep 30;115(3):834–840. doi: 10.1016/s0006-291x(83)80010-2. [DOI] [PubMed] [Google Scholar]
- Halldorsson H., Gray D. A., Shall S. Poly (ADP-ribose) polymerase activity in nucleotide permeable cells. FEBS Lett. 1978 Jan 15;85(2):349–352. doi: 10.1016/0014-5793(78)80489-x. [DOI] [PubMed] [Google Scholar]
- James M. R., Lehmann A. R. Role of poly(adenosine diphosphate ribose) in deoxyribonucleic acid repair in human fibroblasts. Biochemistry. 1982 Aug 17;21(17):4007–4013. doi: 10.1021/bi00260a016. [DOI] [PubMed] [Google Scholar]
- Johnstone A. P. Rejoining of DNA strand breaks is an early nuclear event during the stimulation of quiescent lymphocytes. Eur J Biochem. 1984 Apr 16;140(2):401–406. doi: 10.1111/j.1432-1033.1984.tb08116.x. [DOI] [PubMed] [Google Scholar]
- Johnstone A. P., Williams G. T. Role of DNA breaks and ADP-ribosyl transferase activity in eukaryotic differentiation demonstrated in human lymphocytes. Nature. 1982 Nov 25;300(5890):368–370. doi: 10.1038/300368a0. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Shall S., Whish W. J. The relationship between cell growth, macromolecular synthesis and poly ADP-ribose polymerase in lymphoid cells. Exp Cell Res. 1974 Jan;83(1):63–72. doi: 10.1016/0014-4827(74)90688-0. [DOI] [PubMed] [Google Scholar]
- Purnell M. R., Whish W. J. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem J. 1980 Mar 1;185(3):775–777. doi: 10.1042/bj1850775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochette-Egly C., Ittel M. E., Bilen J., Mandel P. Effect of nicotinamide on RNA and DNA synthesis and on poly(ADP-ribose) polymerase activity in normal and phytohemagglutinin stimulated human lymphocytes. FEBS Lett. 1980 Oct 20;120(1):7–11. doi: 10.1016/0014-5793(80)81033-7. [DOI] [PubMed] [Google Scholar]
- Rutherford T. R., Clegg J. B., Weatherall D. J. K562 human leukaemic cells synthesise embryonic haemoglobin in response to haemin. Nature. 1979 Jul 12;280(5718):164–165. doi: 10.1038/280164a0. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Johnstone A. P. ADP-ribosyl transferase, rearrangement of DNA, and cell differentiation. Biosci Rep. 1983 Sep;3(9):815–830. doi: 10.1007/BF01133780. [DOI] [PubMed] [Google Scholar]
- Winterbourne D. J., Mora P. T. Altered metabolism of heparan sulfate in simian virus 40 transformed cloned mouse cells. J Biol Chem. 1978 Jul 25;253(14):5109–5120. [PubMed] [Google Scholar]


