Abstract
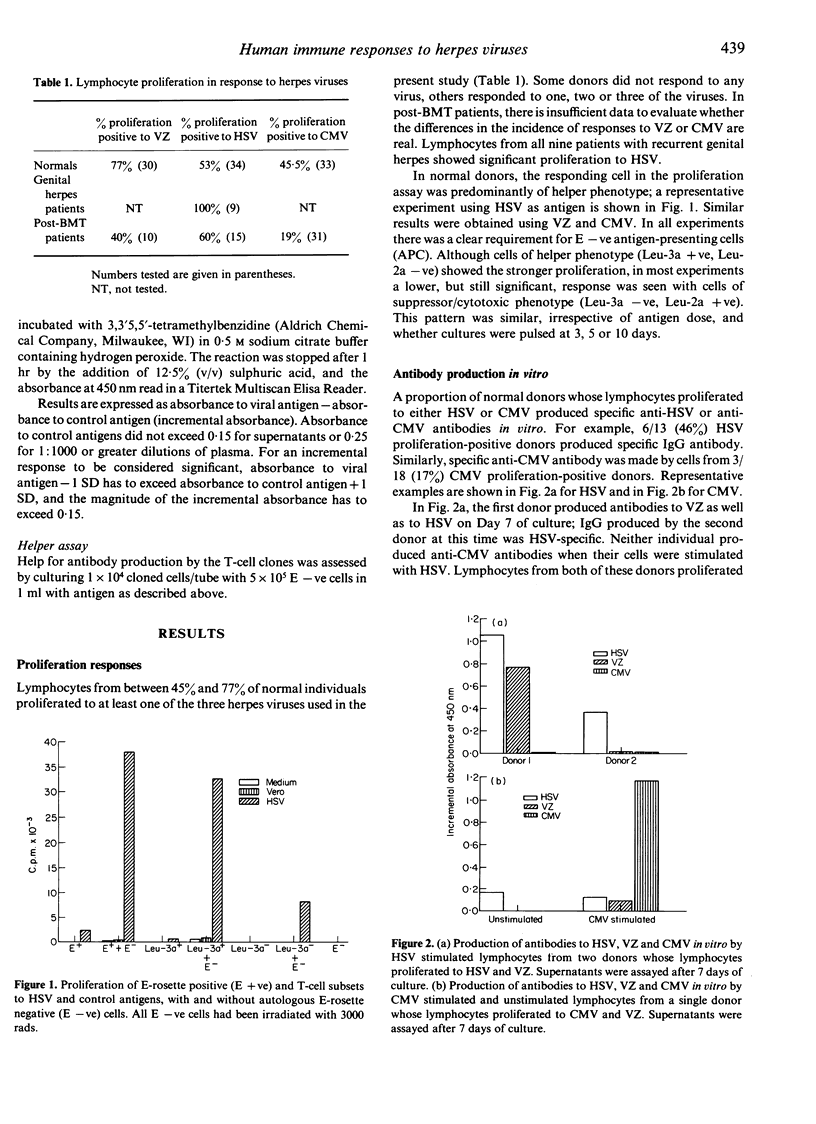
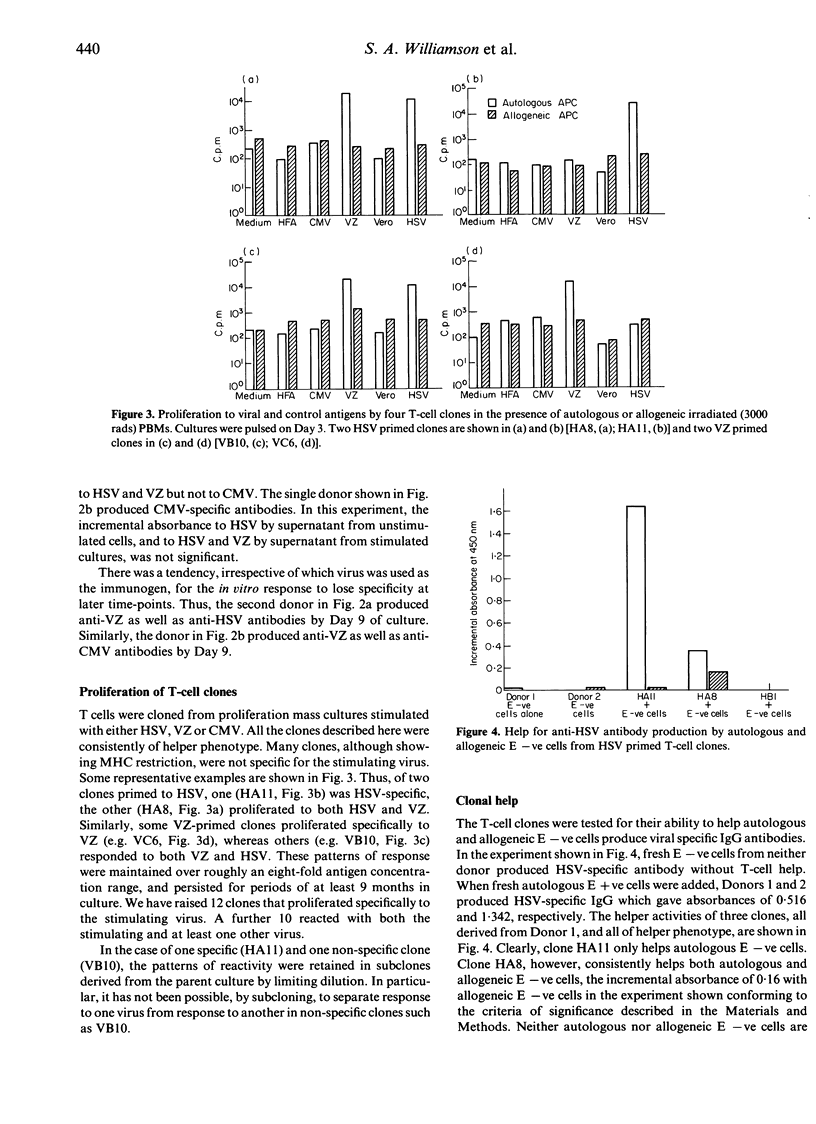
The cell principally responsible for lymphocyte proliferation to herpes simplex virus (HSV), varicella-zoster (VZ) and cytomegalovirus (CMV) has been shown to be a T cell of helper phenotype. Lymphocytes from a proportion of proliferation-positive normal individuals produced anti-viral antibody in vitro. Although in some cases, and at some time-points, the antibody was specific for the priming virus, in others, antibodies to more than one virus were detected. Similarly, some T-cell clones proliferated specifically to the priming virus, whereas others were not specific for the virus used in the priming culture. Two clones helped the production of HSV-specific antibody, one by autologous, the other by both autologous and allogeneic non-T cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berle E. J., Jr, Thorsby E. The proliferative T cell response to herpes simplex virus (HSV) antigen is restricted by self HLA-D. Clin Exp Immunol. 1980 Mar;39(3):668–675. [PMC free article] [PubMed] [Google Scholar]
- Callard R. E., Smith C. M. Histocompatibility requirements for T cell help in specific in vitro antibody responses to influenza virus by human blood lymphocytes. Eur J Immunol. 1981 Mar;11(3):206–212. doi: 10.1002/eji.1830110309. [DOI] [PubMed] [Google Scholar]
- Callard R. E. Specific in vitro antibody response to influenza virus by human blood lymphocytes. Nature. 1979 Dec 13;282(5740):734–736. doi: 10.1038/282734a0. [DOI] [PubMed] [Google Scholar]
- Dix R. D., Pereira L., Baringer J. R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981 Oct;34(1):192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckels D. D., Lake P., Lamb J. R., Johnson A. H., Shaw S., Woody J. N., Hartzman R. J. SB-restricted presentation of influenza and herpes simplex virus antigens to human T-lymphocyte clones. Nature. 1983 Feb 24;301(5902):716–718. doi: 10.1038/301716a0. [DOI] [PubMed] [Google Scholar]
- Honess R. W. Herpes simplex and 'the herpes complex': diverse observations and a unifying hypothesis. The eighth Fleming lecture. J Gen Virol. 1984 Dec;65(Pt 12):2077–2107. doi: 10.1099/0022-1317-65-12-2077. [DOI] [PubMed] [Google Scholar]
- Hutt-Fletcher L. M., Balachandran N., Elkins M. H. B cell activation by cytomegalovirus. J Exp Med. 1983 Dec 1;158(6):2171–2176. doi: 10.1084/jem.158.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimo K. O., Joronen I. A., Havu V. K. Cell-mediated immunity against herpes simplex virus envelope, capsid, excreted, and crude antigens. Infect Immun. 1983 Jan;39(1):24–28. doi: 10.1128/iai.39.1.24-28.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Lake P., Johnson A. H., Hartzman R. J., Woody J. N. Antigen-specific human T lymphocyte clones: induction, antigen specificity, and MHC restriction of influenza virus-immune clones. J Immunol. 1982 Jan;128(1):233–238. [PubMed] [Google Scholar]
- Lamb J. R., Woody J. N., Hartzman R. J., Eckels D. D. In vitro influenza virus-specific antibody production in man: antigen-specific and HLA-restricted induction of helper activity mediated by cloned human T lymphocytes. J Immunol. 1982 Oct;129(4):1465–1470. [PubMed] [Google Scholar]
- Mitchell D. M., Fitzharris P., Knight R. A., Schild G. C. Kinetics of specific in vitro antibody production following influenza immunization. Clin Exp Immunol. 1982 May;48(2):491–498. [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Gell P. G. Membrane phenotype of murine effector and suppressor T cells involved in delayed hypersensitivity and protective immunity to herpes simplex virus. Cell Immunol. 1983 Feb 1;75(2):348–355. doi: 10.1016/0008-8749(83)90332-5. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Esber E., Saral R., Manischewitz J. F., Rogers J. L., Rook A. H., Santos G. W., Burns W. H. HLA-restricted cytotoxic T lymphocyte and nonthymic cytotoxic lymphocyte responses to cytomegalovirus infection of bone marrow transplant recipients. J Immunol. 1981 May;126(5):2036–2041. [PubMed] [Google Scholar]
- Roberts P. L., Duncan B. E., Raybould T. J., Watson D. H. Purification of herpes simplex virus glycoproteins B and C using monoclonal antibodies and their ability to protect mice against lethal challenge. J Gen Virol. 1985 May;66(Pt 5):1073–1085. doi: 10.1099/0022-1317-66-5-1073. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Subak Sharpe J. H., Ferry P. Antigenic relationship of varicella-zoster and herpes simplex. Lancet. 1965 Oct 9;2(7415):708–711. doi: 10.1016/s0140-6736(65)90452-6. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., Magoffin R. L. Immunological relationship between herpes simplex and varicella-zoster viruses demonstrated by complement-fixation, neutralization and fluorescent antibody tests. J Gen Virol. 1969 Apr;4(3):321–328. doi: 10.1099/0022-1317-4-3-321. [DOI] [PubMed] [Google Scholar]
- Sethi K. K., Omata Y., Schneweis K. E. Protection of mice from fatal herpes simplex virus type 1 infection by adoptive transfer of cloned virus-specific and H-2-restricted cytotoxic T lymphocytes. J Gen Virol. 1983 Feb;64(Pt 2):443–447. doi: 10.1099/0022-1317-64-2-443. [DOI] [PubMed] [Google Scholar]
- Souhami R. L., Babbage J., Callard R. E. Specific in vitro antibody response to varicella zoster. Clin Exp Immunol. 1981 Oct;46(1):98–105. [PMC free article] [PubMed] [Google Scholar]
- Souhami R. L., Babbage J., Sigfusson A. Defective in vitro antibody production to Varicella zoster and other virus antigens in patients with Hodgkin's disease. Clin Exp Immunol. 1983 Aug;53(2):297–307. [PMC free article] [PubMed] [Google Scholar]
- Ten Napel C. H., The T. H., Bijker J., De Gast G. C., Langenhuysen M. M. Cytomegalovirus-directed lymphocyte reactivity in healthy adults tested by a CMV-induced lymphocyte transformation test. Clin Exp Immunol. 1977 Jul;29(1):52–60. [PMC free article] [PubMed] [Google Scholar]
- The T. H., Langenhuysen M. M. Antibodies against membrane antigens of cytomegalovirus infected cells in sera of patients with a cytomegalovirus infection. Clin Exp Immunol. 1972 Aug;11(4):475–482. [PMC free article] [PubMed] [Google Scholar]
- Yap K. L., Ada G. L., McKenzie I. F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978 May 18;273(5659):238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. I. Lysis restricted by HLA class II MB and DR antigens. J Immunol. 1984 Jul;133(1):422–427. [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. II. Bifunctional clones with cytotoxic and virus-induced proliferative activities exhibit herpes simplex virus type 1 and 2 specific or type common reactivities. J Immunol. 1984 Nov;133(5):2736–2742. [PubMed] [Google Scholar]
- ten Napel C. H., The T. H. Acute cytomegalovirus infection and the host immune response. I. Development and maintenance of cytomegalovirus (CMV) induced in vitro lymphocyte reactivity and its relationship to the production of CMV antibodies. Clin Exp Immunol. 1980 Feb;39(2):263–271. [PMC free article] [PubMed] [Google Scholar]


