Abstract
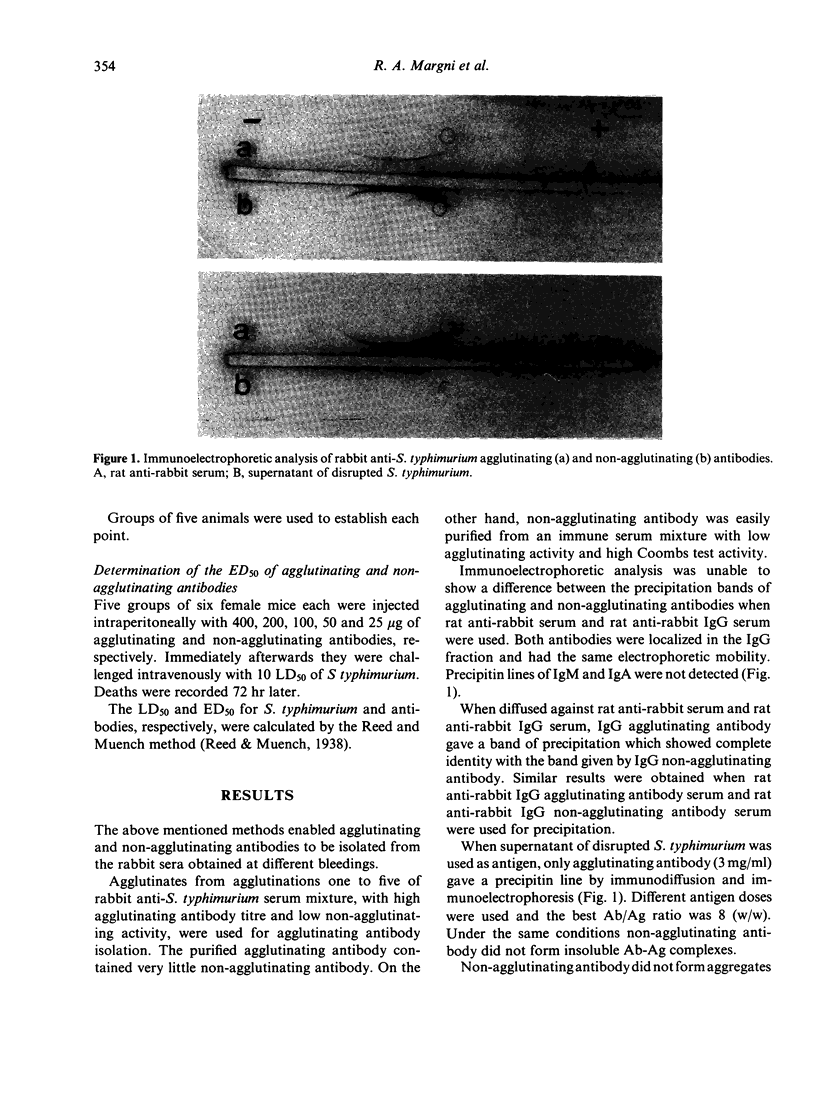
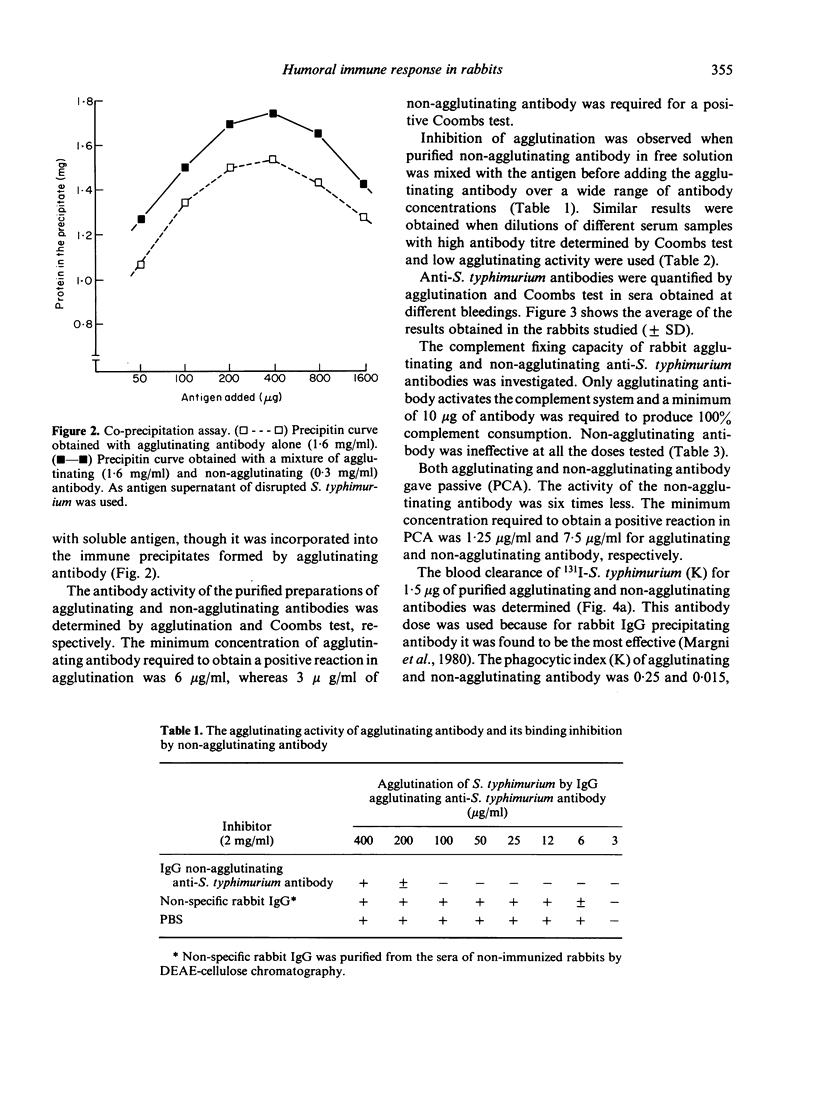
Agglutinating and non-agglutinating anti-Salmonella typhimurium antibodies were specifically purified from the sera of immunized rabbits. Both types of antibody had the same electrophoretic mobility and were localized in the IgG fraction. It was not possible to find antigenic differences between agglutinating and non-agglutinating antibodies by immunodiffusion.
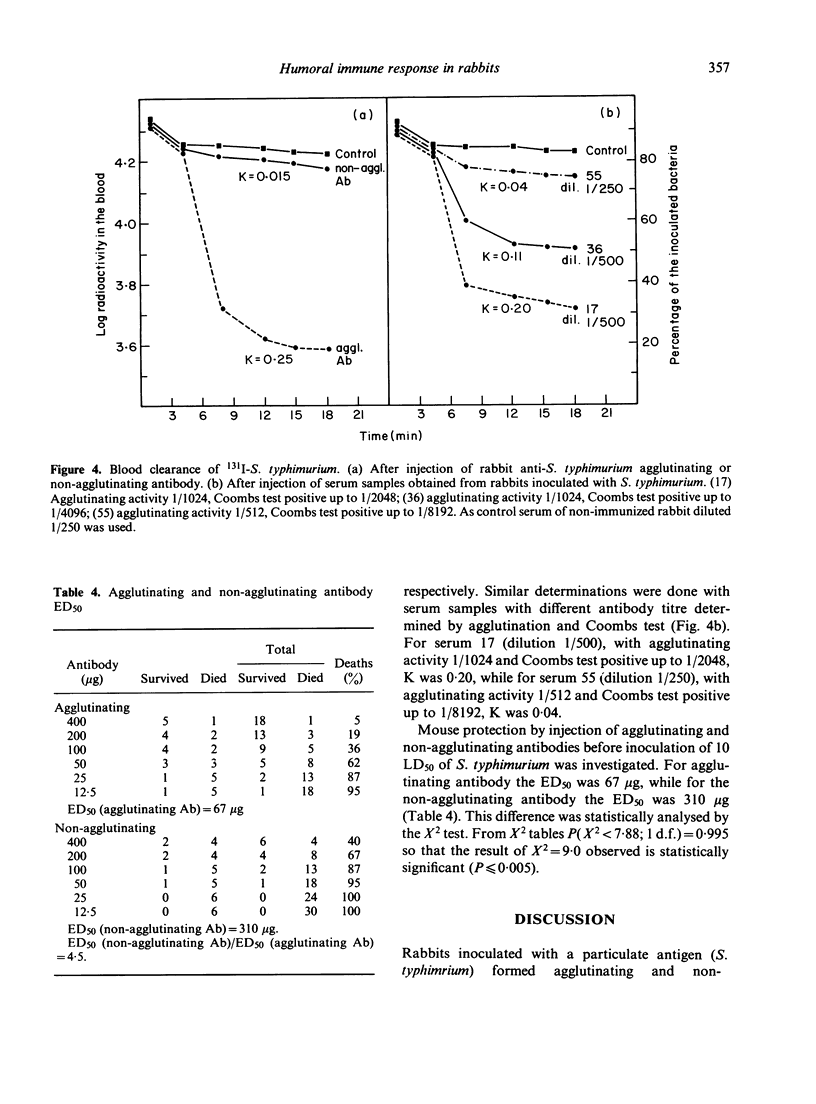
Agglutinating antibody activated the complement system, while non-agglutinating antibody lacked this capacity. Only the former increased clearance of antigen from the blood. When serum samples with different antibody titres determined by agglutination (agglutinating antibody) and Coombs test (non-agglutinating antibody) were injected in mice, clearance of antigen from the blood showed changes. These results were similar to those previously observed by us when different precipitating: co-precipitating antibody ratios were used, and indicated that competition of both antibodies for the antigen depends on their respective amounts.
When mice protection tests were set up by injection of agglutinating and non-agglutinating antibody before the inoculation of 10 LD50 S. typhimurium, non-agglutinating antibody was found to be less effective than agglutinating antibody.
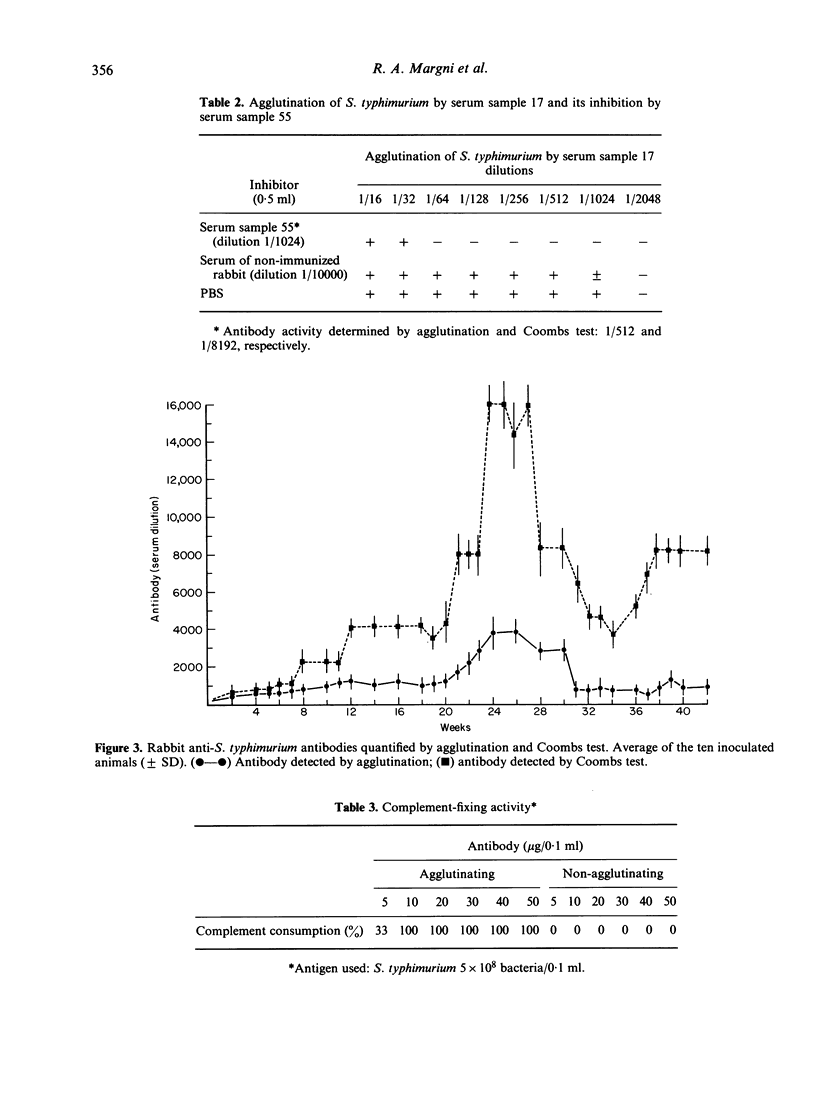
Non-agglutinating antibody was detectable during the whole course of immunization. Its serum concentration was higher than that of the agglutinating antibody.
Non-agglutinating antibody behaves in a similar way to co-precipitating antibody. The initially proposed hypothesis that such antibodies could interfere with immunity to certain chronic infections was extended to include the non-agglutinating antibodies demonstrated here.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cordal M. E., Margni R. A. Isolation, purification and biological properties of horse precipitating and non precipitating antibodies. Immunochemistry. 1974 Dec;11(12):765–770. doi: 10.1016/0019-2791(74)90295-x. [DOI] [PubMed] [Google Scholar]
- Forget A., Borduas A. G. An immunological enhancement phenomenon in experimental brucella infection of the chicks. Int Arch Allergy Appl Immunol. 1977;53(2):190–194. doi: 10.1159/000231751. [DOI] [PubMed] [Google Scholar]
- Hajos S. E., Carbonetto C., Margni R. A., Esteva M., Segura E. L. Purification and properties of anti-Trypanosoma cruzi antibodies isolated from patients with chronic Chagas' disease. Immunol Lett. 1982 Apr;4(4):199–203. doi: 10.1016/0165-2478(82)90014-1. [DOI] [PubMed] [Google Scholar]
- Margni R. A., Cordal M. E., Leoni J., Hajos S. E., Veira S., Manghi M., Bazzurro M. Non-precipitating antibodies isolated by immunoadsorption. Immunochemistry. 1977 Apr;14(4):299–303. doi: 10.1016/0019-2791(77)90253-1. [DOI] [PubMed] [Google Scholar]
- Margni R. A., Hajos S. Biological and physicochemical properties of purified anti-DNP guinea-pig non-precipitating antibodies. Immunology. 1973 Mar;24(3):435–443. [PMC free article] [PubMed] [Google Scholar]
- Margni R. A., Paz C. B., Cordal M. E. Immunochemical behavior of sheep non-precipitating antibodies isolated by immunoadsorption. Immunochemistry. 1976 Mar;13(3):209–214. doi: 10.1016/0019-2791(76)90217-2. [DOI] [PubMed] [Google Scholar]
- Margni R. A., Perdigón G., Abatángelo C., Gentile T., Binaghi R. A. Immunobiological behaviour of rabbit precipitating and non-precipitating (co-precipitating) antibodies. Immunology. 1980 Nov;41(3):681–686. [PMC free article] [PubMed] [Google Scholar]
- Margni R., Binaghi R. Purification and properties of non-precipitating rabbit antibodies. Immunology. 1972 Apr;22(4):557–563. [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. A., Katzenstein D., Norquist D., Chikami G., Wunderlich A., Braude A. I. Role of blocking antibody in disseminated gonococcal infection. J Immunol. 1978 Nov;121(5):1884–1888. [PubMed] [Google Scholar]
- OVARY Z. [Cutaneous anaphylaxis in the albino rat]. Int Arch Allergy Appl Immunol. 1952;3(4):293–301. doi: 10.1159/000227977. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdigón G., Margni R. A., Gentile T., Abatángelo C., Dokmetjian J. Human anti-tetanus toxin precipitating and co-precipitating antibodies. Immunology. 1982 Jan;45(1):183–190. [PMC free article] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]



