Abstract
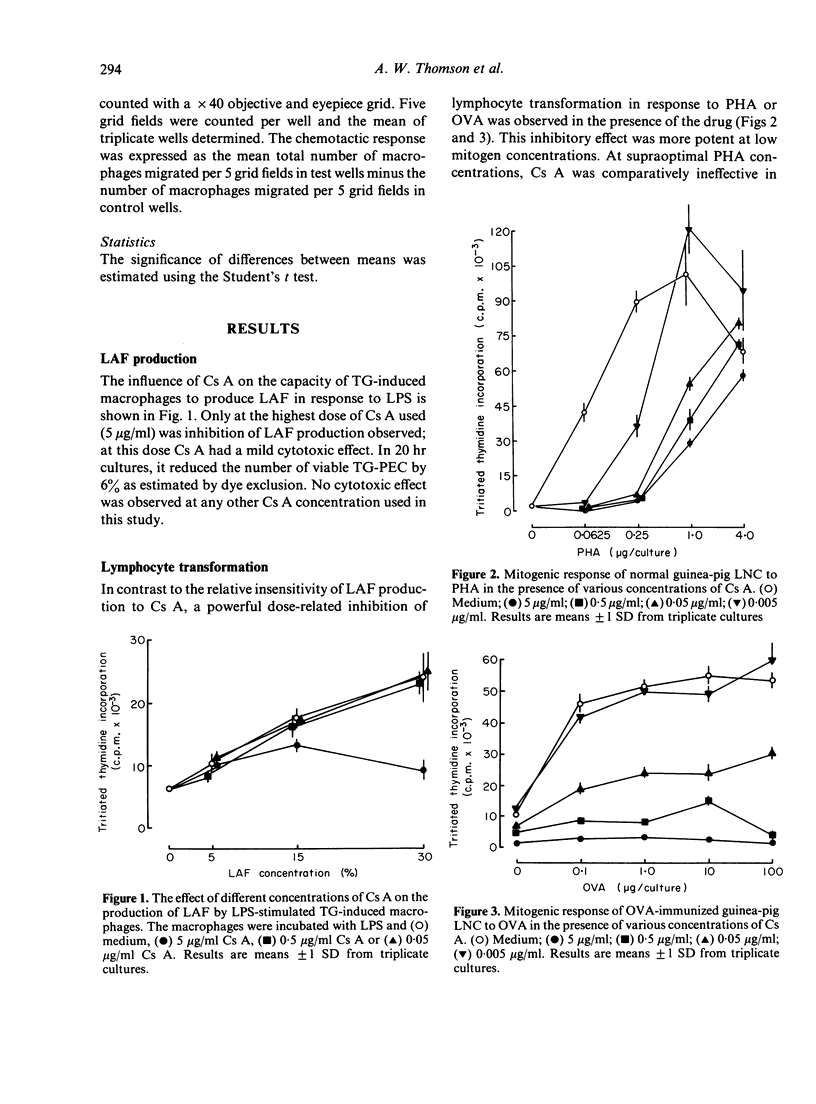
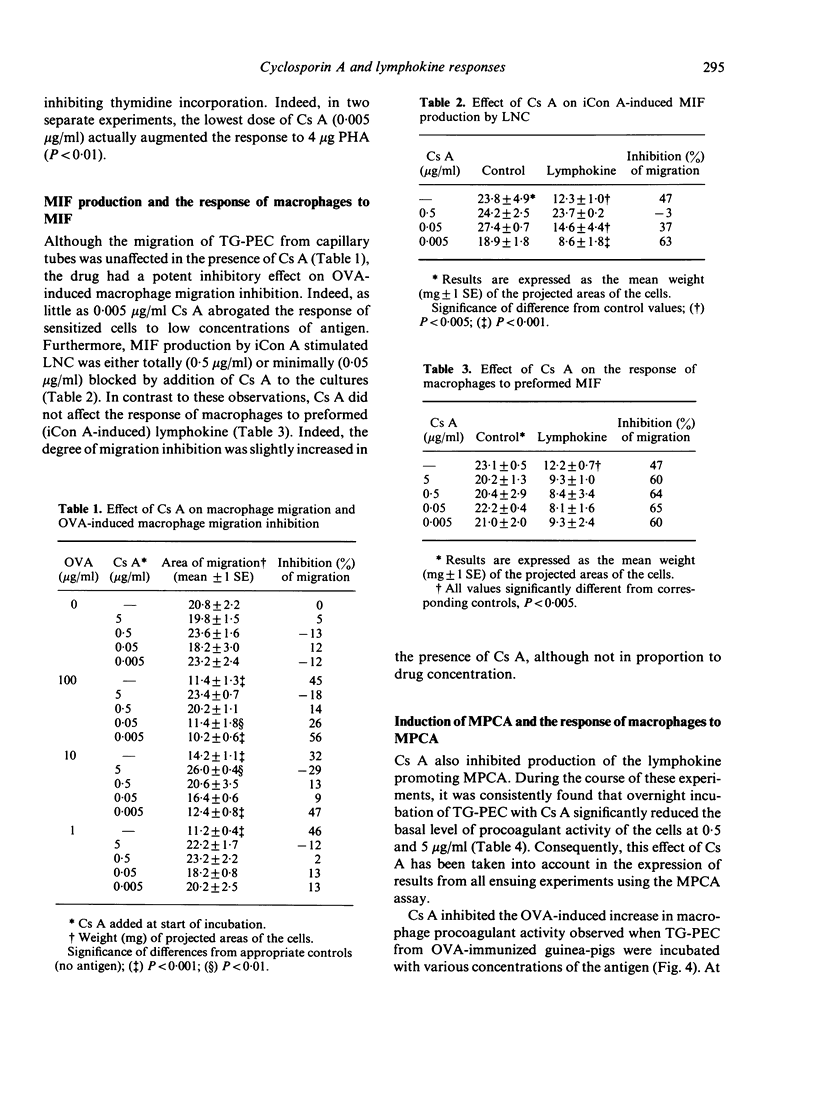
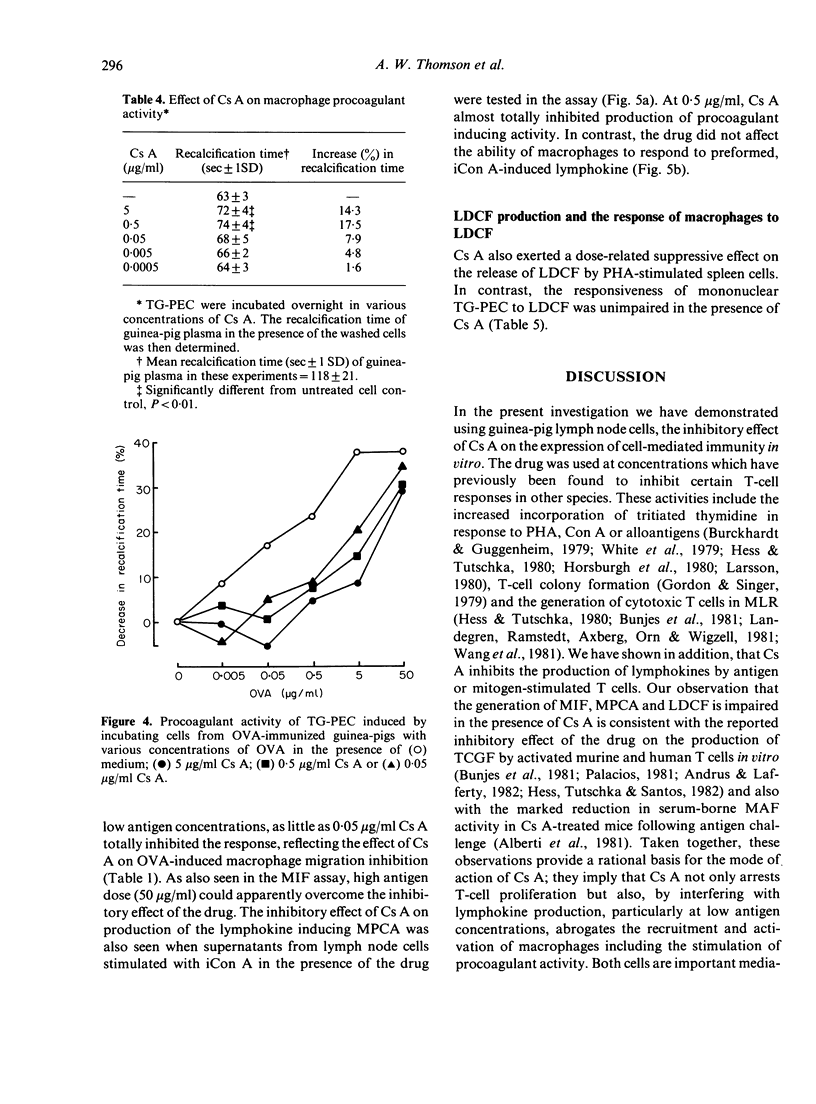
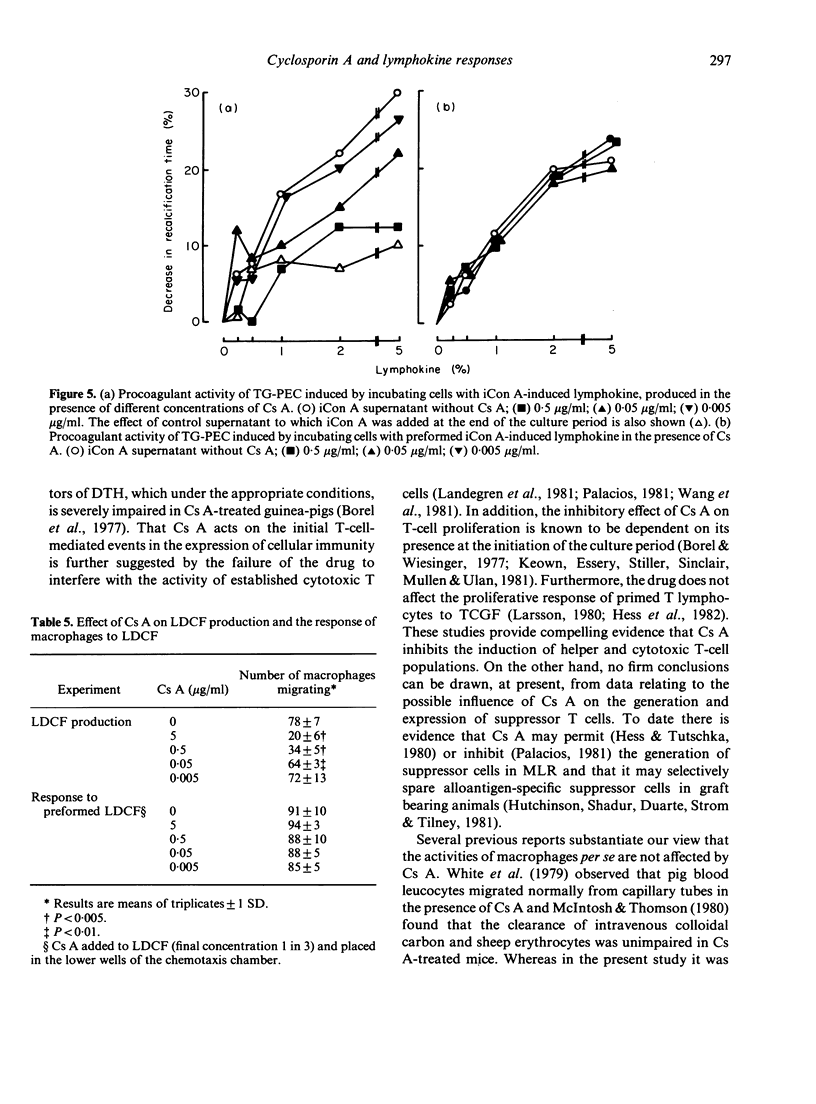
Cyclosporin A (Cs A) exerted a dose-related inhibitory effect on antigen (ovalbumin, OVA) and phytohaemagglutinin (PHA)-induced transformation of guinea-pig lymph node cells (LNC). Whereas 0.05 micrograms/ml was sufficient to depress these responses markedly, it required 100-fold this concentration of Cs A to inhibit the production of lymphocyte activating factor (LAF) by lipopolysaccharide (LPS) stimulated peritoneal macrophages. Addition of Cs A together with insoluble concanavalin A (iCon A) to LNC cultures resulted in suppressed lymphokine production, as assessed by measurement of migration inhibition factor (MIF), the generation of macrophage procoagulant activity (MPCA) and the release of lymphocyte-derived-macrophage chemotactic factor (LDCF). Cs A also inhibited MIF and procoagulant production by sensitized peritoneal exudate cells in response to antigen, at the same concentrations which blocked lymphocyte transformation. In contrast, Cs A had no direct effect on the migration of peritoneal cells from capillary tubes, or on the responses of macrophages to preformed MIF, the lymphokine inducing MPCA or LDCF. Overnight incubation of macrophages with Cs A did, however, result in mild inhibition of their basal level of procoagulant activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti S., Boraschi D., Luini W., Tagliabue A. Effects of in vivo treatments with cyclosporin-A on mouse cell-mediated immune responses. Int J Immunopharmacol. 1981;3(4):357–364. doi: 10.1016/0192-0561(81)90031-x. [DOI] [PubMed] [Google Scholar]
- Andrus L., Lafferty K. J. Inhibition of T-cell activity by cyclosporin A. Scand J Immunol. 1981 May;15(5):449–458. doi: 10.1111/j.1365-3083.1982.tb00670.x. [DOI] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Bunjes D., Hardt C., Röllinghoff M., Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981 Aug;11(8):657–661. doi: 10.1002/eji.1830110812. [DOI] [PubMed] [Google Scholar]
- Burckhardt J. J., Guggenheim B. Cyclosporin A: in vivo and in vitro suppression of rat T-lymphocyte function. Immunology. 1979 Apr;36(4):753–757. [PMC free article] [PubMed] [Google Scholar]
- Calderon J., Kiely J. M., Lefko J. L., Unanue E. R. The modulation of lymphocyte functions by molecules secreted by macrophages. I. Description and partial biochemical analysis. J Exp Med. 1975 Jul 1;142(1):151–164. doi: 10.1084/jem.142.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Friedrich W., Lazary S., Geczy C., de Weck A. L. Lymphokines. I. Use of insoluble concanavalin A for the production of migration inhibitory factor in guinea pig lymphocyte cultures. Int Arch Allergy Appl Immunol. 1975;49(4):504–518. [PubMed] [Google Scholar]
- Geczy C. L., Hopper K. E. A mechanism of migration inhibition in delayed-type hypersensitivity reactions. II. Lymphokines promote procoagulant activity of macrophages in vitro. J Immunol. 1981 Mar;126(3):1059–1065. [PubMed] [Google Scholar]
- Green C. J. Immunosuppression with cyclosporin A: a review. Diagn Histopathol. 1981 Apr-Jun;4(2):157–174. [PubMed] [Google Scholar]
- Hess A. D., Tutschka P. J. Effect of cyclosporin A on human lymphocyte responses in vitro. I. CsA allows for the expression of alloantigen-activated suppressor cells while preferentially inhibiting the induction of cytolytic effector lymphocytes in MLR. J Immunol. 1980 Jun;124(6):2601–2608. [PubMed] [Google Scholar]
- Hess A. D., Tutschka P. J., Santos G. W. Effect of cyclosporin A on human lymphocyte responses in vitro. III. CsA inhibits the production of T lymphocyte growth factors in secondary mixed lymphocyte responses but does not inhibit the response of primed lymphocytes to TCGF. J Immunol. 1982 Jan;128(1):355–359. [PubMed] [Google Scholar]
- Horsburgh T., Wood P., Brent L. Suppression of in vitro lymphocyte reactivity by cyclosporin A: existence of a population of drug-resistant cytotoxic lymphocytes. Nature. 1980 Aug 7;286(5773):609–611. doi: 10.1038/286609a0. [DOI] [PubMed] [Google Scholar]
- Hutchinson I. F., Shadur C. A., Duarte J. S., Strom T. B., Tilney N. L. Cyclosporin A spares selectively lymphocytes with donor-specific suppressor characteristics. Transplantation. 1981 Sep;32(3):210–216. doi: 10.1097/00007890-198109000-00006. [DOI] [PubMed] [Google Scholar]
- Keown P. A., Essery G. L., Stiller C. R., Sinclair N. R., Mullen R., Ulan R. A. Mechanisms of immunosuppression by cyclosporin. Transplant Proc. 1981 Mar;13(1 Pt 1):386–389. [PubMed] [Google Scholar]
- Landegren U., Ramstedt U., Axberg I., Orn A., Wigzell H. Cyclosporin a permits the distinction between specific T and NK activity generated in a human MLC. Int J Cancer. 1981 Dec;28(6):725–730. doi: 10.1002/ijc.2910280611. [DOI] [PubMed] [Google Scholar]
- Larsson E. L. Cyclosporin A and dexamethasone suppress T cell responses by selectively acting at distinct sites of the triggering process. J Immunol. 1980 Jun;124(6):2828–2833. [PubMed] [Google Scholar]
- McIntosh L. C., Thompson A. W. Activity of the mononuclear phagocyte system in cyclosporin A-treated mice. Transplantation. 1980 Nov;30(5):384–386. doi: 10.1097/00007890-198011000-00017. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Jones E. E., Boetcher D. A. Increased chemotactic responses of macrophages from BCG-infected mice. Cell Immunol. 1975 May;17(1):268–276. doi: 10.1016/s0008-8749(75)80026-8. [DOI] [PubMed] [Google Scholar]
- Palacios R. Cyclosporin A inhibits the proliferative response and the generation of helper, suppressor and cytotoxic T-cell functions in the autologous mixed lymphocyte reaction. Cell Immunol. 1981 Jul 1;61(2):453–462. doi: 10.1016/0008-8749(81)90393-2. [DOI] [PubMed] [Google Scholar]
- Palacios R., Möller G. Cyclosporin A blocks receptors for HLA-DR antigens on T cells. Nature. 1981 Apr 30;290(5809):792–794. doi: 10.1038/290792a0. [DOI] [PubMed] [Google Scholar]
- Ryffel B., Donatsch P., Götz U., Tschopp M. Cyclosporin receptor on mouse lymphocytes. Immunology. 1980 Dec;41(4):913–919. [PMC free article] [PubMed] [Google Scholar]
- Wang B. S., Heacock E. H., Collins K. H., Hutchinson I. F., Tilney N. L., Mannick J. A. Suppressive effects of cyclosporin A on the induction of alloreactivity in vitro and in vivo. J Immunol. 1981 Jul;127(1):89–93. [PubMed] [Google Scholar]


