Abstract
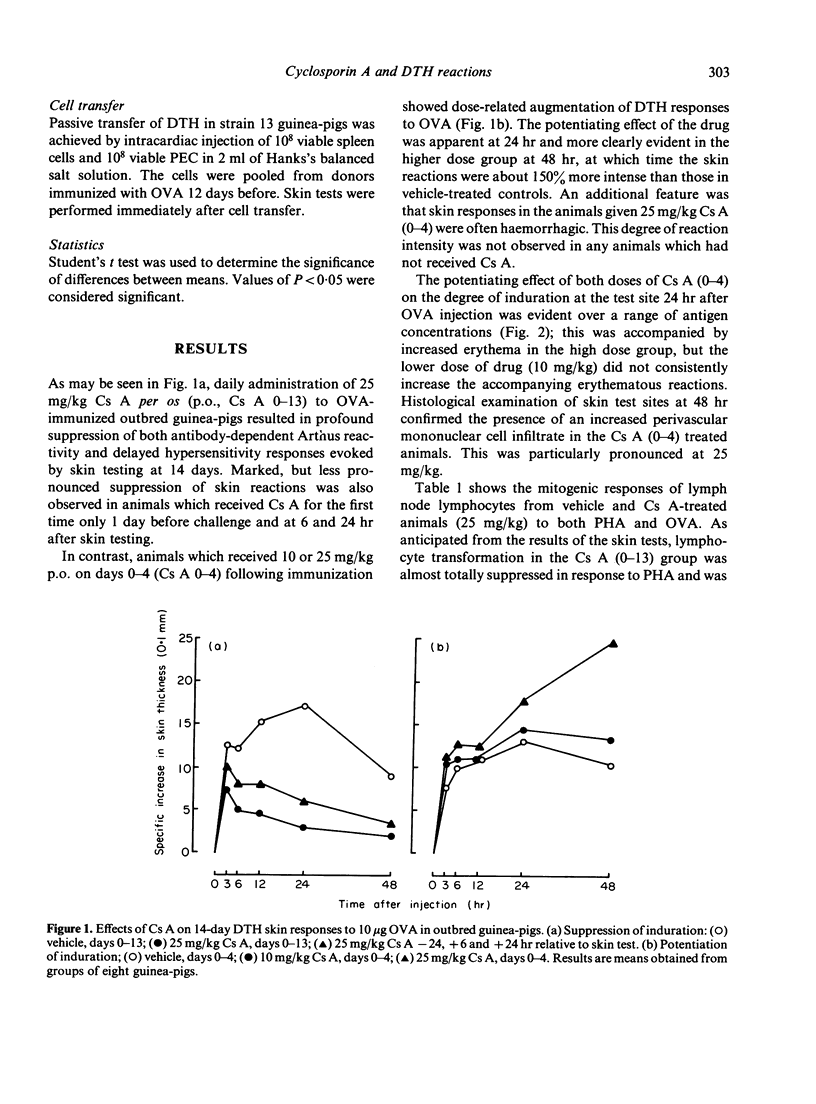
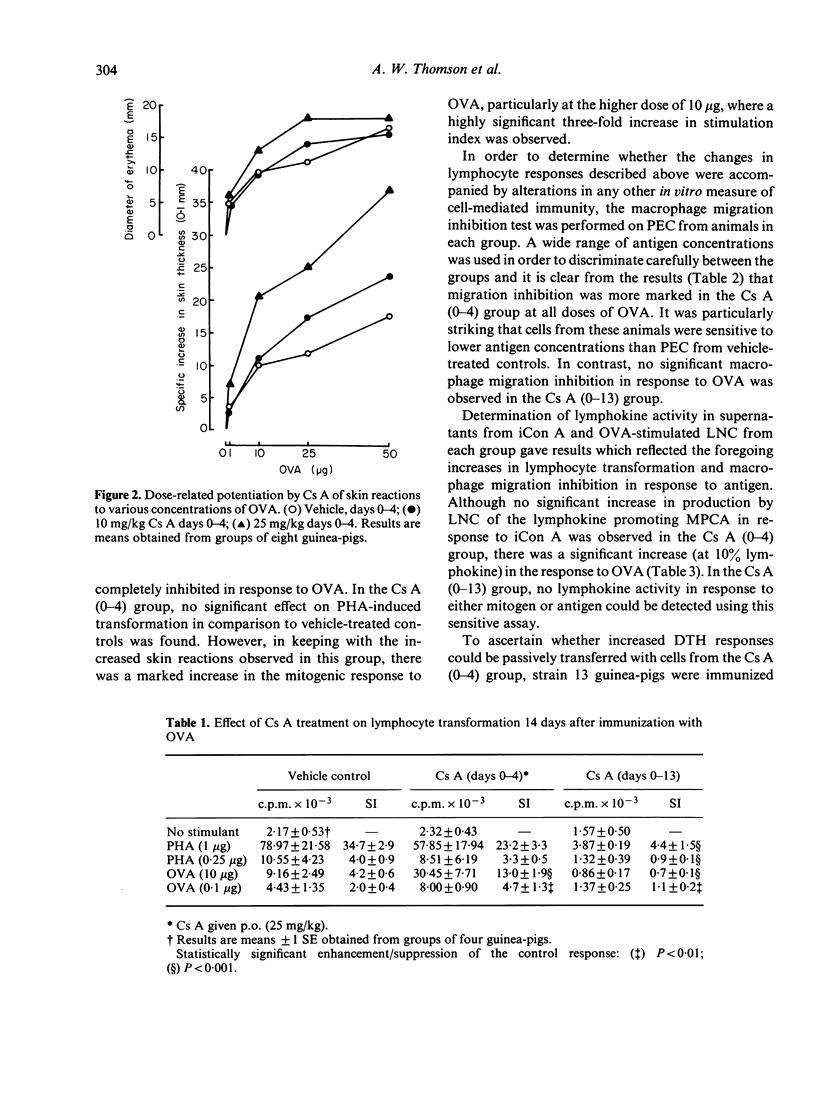
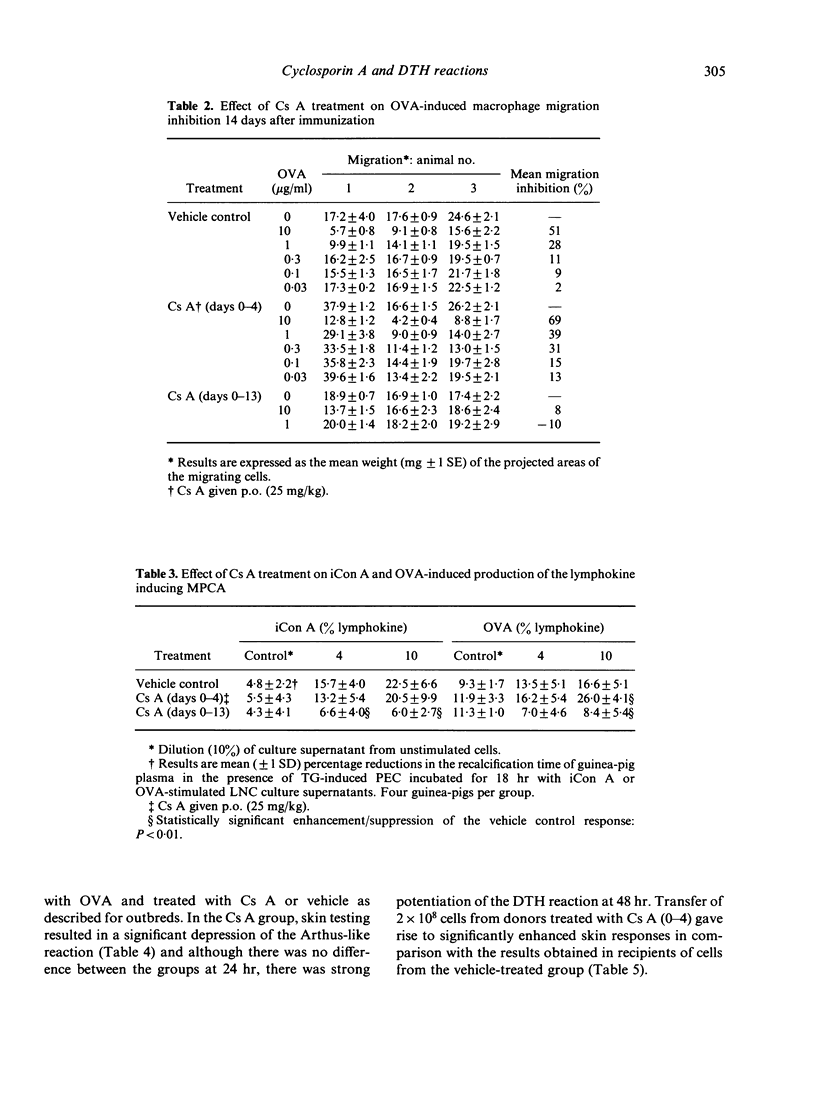
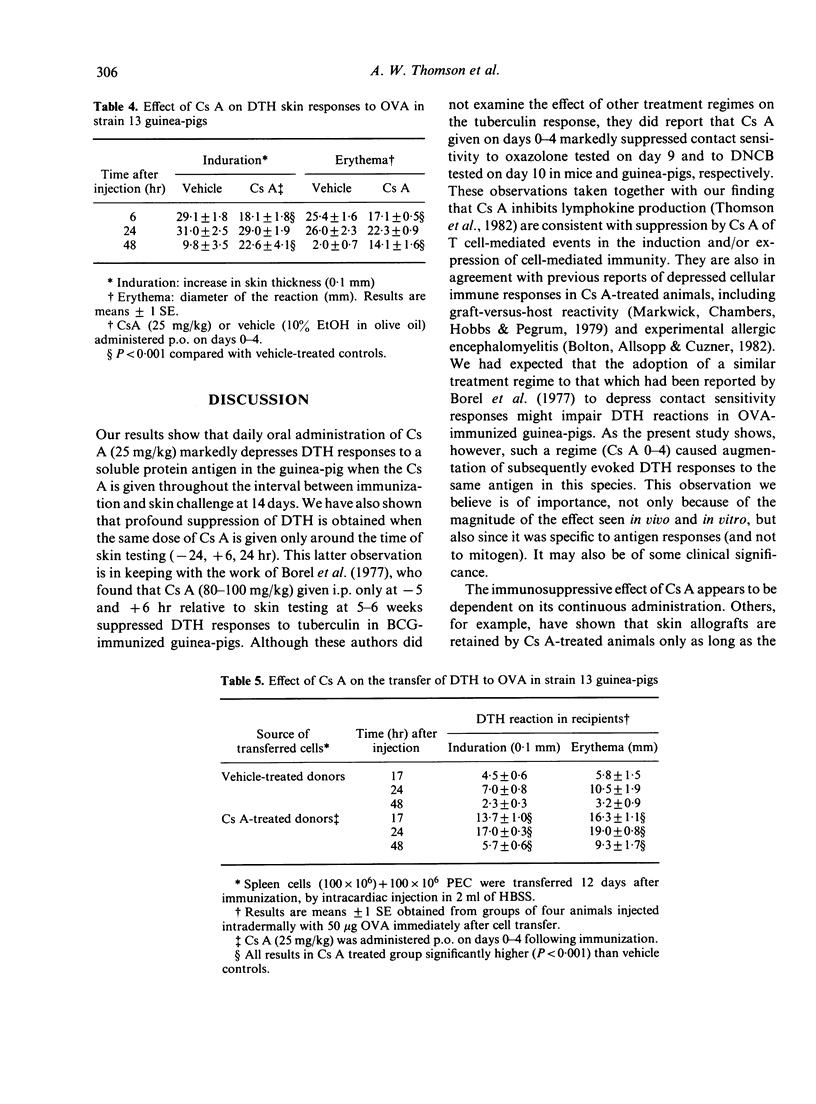
Cyclosporin A (Cs A) administered daily (25 mg/kg per os) to outbred guinea-pigs for 2 weeks following immunization with ovalbumin (OVA; CsA 0-13) caused profound suppression of 14-day delayed-type hypersensitivity (DTH) skin reactions. Very marked impairment of DTH was also found when Cs A was given for the first time 24 hr before skin testing and at 6 and 24 hr thereafter. In contrast, Cs A given on days 0-4 following OVA immunization (Cs A 0-4) caused dose-related potentiation of 14-day skin responses. These changes in the magnitude and character of DTH in vivo were accompanied by striking alterations in lymphocyte transformation responses and in the extent of macrophage migration inhibition and lymphokine production. Whereas Cs A (0-13) caused almost total suppression of the mitogenic responses of lymph node cells to PHA and antigen, OVA-induced migration inhibition and production of the lymphokine inducing macrophage procoagulant activity (MPCA), Cs A (0-4) augmented these responses to OVA, but did not affect lymphocyte transformation or lymphokine production in response to mitogen. Strain 13 guinea-pigs treated with Cs A (0-4) showed depressed Arthus, but augmented DTH responses to OVA. This significant increase in cell-mediated immunity could be passively transferred using spleen and peritoneal exudate cells, suggesting that under these circumstances Cs A (0-4) may interfere with the generation of a population of suppressor cells which regulate DTH reactions in the guinea-pig.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Zembala M. Suppressor T cells in cell-mediated immunity. Br Med Bull. 1976 May;32(2):158–164. doi: 10.1093/oxfordjournals.bmb.a071349. [DOI] [PubMed] [Google Scholar]
- Askenase P. W., Hayden B. J., Gershon R. K. Augmentation of delayed-type hypersensitivity by doses of cyclophosphamide which do not affect antibody responses. J Exp Med. 1975 Mar 1;141(3):697–702. doi: 10.1084/jem.141.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton C., Allsopp G., Cuzner M. L. The effect of cyclosporin A on the adoptive transfer of experimental allergic encephalomyelitis in the Lewis rat. Clin Exp Immunol. 1982 Jan;47(1):127–132. [PMC free article] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Gubler H. U., Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976 Jul;6(4):468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Borel J. F., Meszaros J. Skin transplantation in mice and dogs. Effect of cyclosporin A and dihydrocyclosporin C. Transplantation. 1980 Feb;29(2):161–162. [PubMed] [Google Scholar]
- Bueding E., Hawkins J., Cha Y. N. Antischistosomal effects of cyclosporin A. Agents Actions. 1981 Jul;11(4):380–383. doi: 10.1007/BF01982474. [DOI] [PubMed] [Google Scholar]
- Burckhardt J. J., Guggenheim B. Cyclosporin A: in vivo and in vitro suppression of rat T-lymphocyte function. Immunology. 1979 Apr;36(4):753–757. [PMC free article] [PubMed] [Google Scholar]
- Deeg H. J., Storb R., Gerhard-Miller L., Shulman H. M., Weiden P. L., Thomas E. D. Cyclosporin A, a powerful immunosuppressant in vivo and in vitro in the dog, fails to induce tolerance. Transplantation. 1980 Mar;29(3):230–235. doi: 10.1097/00007890-198003000-00014. [DOI] [PubMed] [Google Scholar]
- Dwyer J. M., Parker D., Turk J. L. Suppression of delayed hypersensitivity to tuberculin by antigenic competition. A positive immunoregulatory mechanism sensitive to cyclophosphamide. Immunology. 1981 Apr;42(4):549–559. [PMC free article] [PubMed] [Google Scholar]
- Geczy C. L., Hopper K. E. A mechanism of migration inhibition in delayed-type hypersensitivity reactions. II. Lymphokines promote procoagulant activity of macrophages in vitro. J Immunol. 1981 Mar;126(3):1059–1065. [PubMed] [Google Scholar]
- Gershon R. K. T cell control of antibody production. Contemp Top Immunobiol. 1974;3:1–40. doi: 10.1007/978-1-4684-3045-5_1. [DOI] [PubMed] [Google Scholar]
- Green C. J., Allison A. C. Extensive prolongation of rabbit kidney allograft survival after short-term cyclosporin-A treatment. Lancet. 1978 Jun 3;1(8075):1182–1183. doi: 10.1016/s0140-6736(78)90970-4. [DOI] [PubMed] [Google Scholar]
- Hess A. D., Tutschka P. J. Effect of cyclosporin A on human lymphocyte responses in vitro. I. CsA allows for the expression of alloantigen-activated suppressor cells while preferentially inhibiting the induction of cytolytic effector lymphocytes in MLR. J Immunol. 1980 Jun;124(6):2601–2608. [PubMed] [Google Scholar]
- Homan W. P., Fabre J. W., Williams K. A., Millard P. R., Morris P. J. Studies on the immunosuppressive properties of cyclosporin a in rats receiving renal allografts. Transplantation. 1980 May;29(5):361–366. doi: 10.1097/00007890-198005000-00003. [DOI] [PubMed] [Google Scholar]
- Huber B., Devinsky O., Gershon R. K., Cantor H. Cell-mediated immunity: delayed-type hypersensitivity and cytotoxic responses are mediated by different T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1534–1539. doi: 10.1084/jem.143.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson I. F., Shadur C. A., Duarte J. S., Strom T. B., Tilney N. L. Cyclosporin A spares selectively lymphocytes with donor-specific suppressor characteristics. Transplantation. 1981 Sep;32(3):210–216. doi: 10.1097/00007890-198109000-00006. [DOI] [PubMed] [Google Scholar]
- Jamieson S. W., Burton N. A., Bieber C. P., Reitz B. A., Oyer P. E., Stinson E. B., Shumway N. E. Cardiac-allograft survival in primates treated with cyclosporin A. Lancet. 1979 Mar 10;1(8115):545–545. doi: 10.1016/s0140-6736(79)90959-0. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Sharrow S. O., Simpson E. T-cell populations with different functions. Nature. 1975 Feb 13;253(5492):544–546. doi: 10.1038/253544a0. [DOI] [PubMed] [Google Scholar]
- Katz S. I., Parker D., Turk J. L. B-cell suppression of delayed hypersensitivity reactions. Nature. 1974 Oct 11;251(5475):550–551. doi: 10.1038/251550a0. [DOI] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Potentiation of T-cell-mediated immunity by selective suppression of antibody formation with cyclophosphamide. J Exp Med. 1974 Jun 1;139(6):1529–1539. doi: 10.1084/jem.139.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lems S. P., Capel P. J., Koene R. A. Rejection of long-surviving mouse skin allografts after withdrawal of cyclosporin A therapy. Transplant Proc. 1980 Jun;12(2):283–286. [PubMed] [Google Scholar]
- Markwick J. R., Chambers J. D., Hobbs J. R., Pegrum G. D. Timing of cyclosporin-A therapy for abrogation of HVG and GVH responses in rats. Lancet. 1979 Nov 17;2(8151):1037–1040. doi: 10.1016/s0140-6736(79)92441-3. [DOI] [PubMed] [Google Scholar]
- Palacios R. Concanavalin A triggers T lymphocytes by directly interacting with their receptors for activation. J Immunol. 1982 Jan;128(1):337–342. [PubMed] [Google Scholar]
- Palacios R. Cyclosporin A inhibits the proliferative response and the generation of helper, suppressor and cytotoxic T-cell functions in the autologous mixed lymphocyte reaction. Cell Immunol. 1981 Jul 1;61(2):453–462. doi: 10.1016/0008-8749(81)90393-2. [DOI] [PubMed] [Google Scholar]
- Silver J., Benacerraf B. Dissociation of T cell helper function and delayed hypersensitivity. J Immunol. 1974 Dec;113(6):1872–1875. [PubMed] [Google Scholar]
- Thomson A. W., Moon D. K., Geczy C. L., Nelson D. S. Cyclosporin A inhibits lymphokine production but not the responses of macrophages to lymphokines. Immunology. 1983 Feb;48(2):291–299. [PMC free article] [PubMed] [Google Scholar]
- White D. J., Plumb A., Calne R. Y. The immune status of transplant recipients immunosuppressed with cyclosporin-A. Transplant Proc. 1981 Sep;13(3):1666–1668. [PubMed] [Google Scholar]


