Abstract
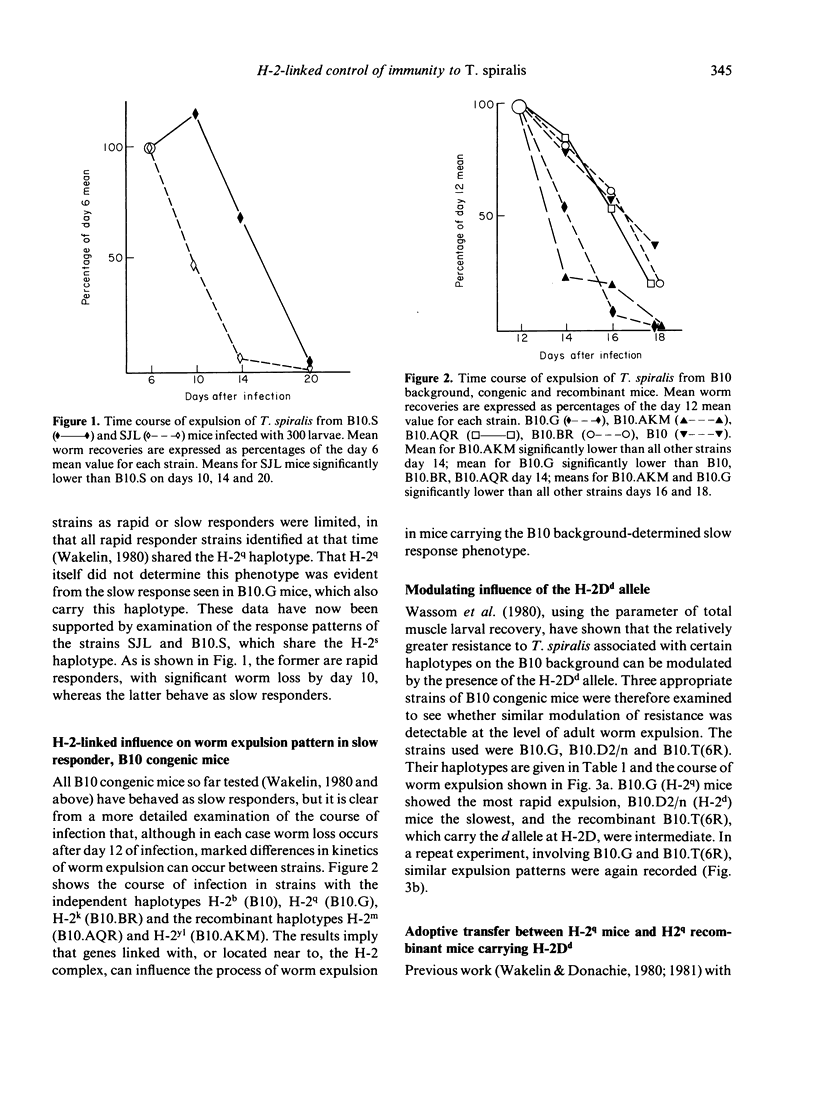
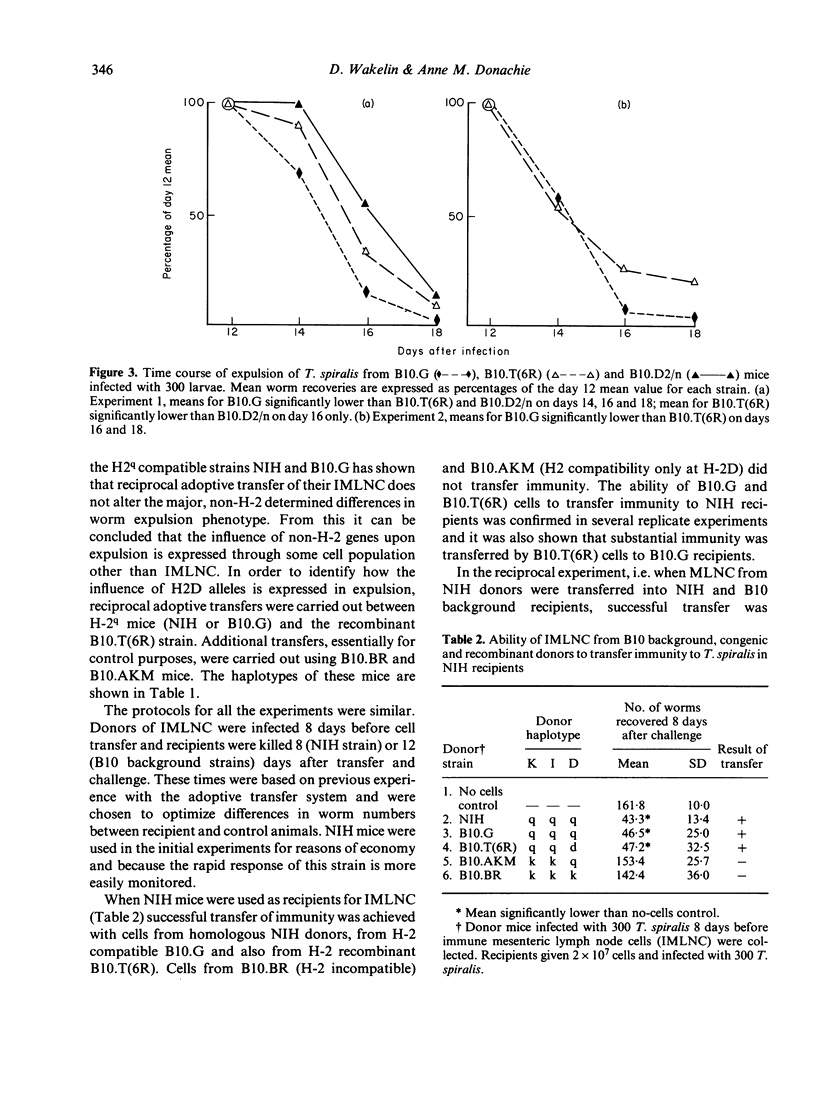
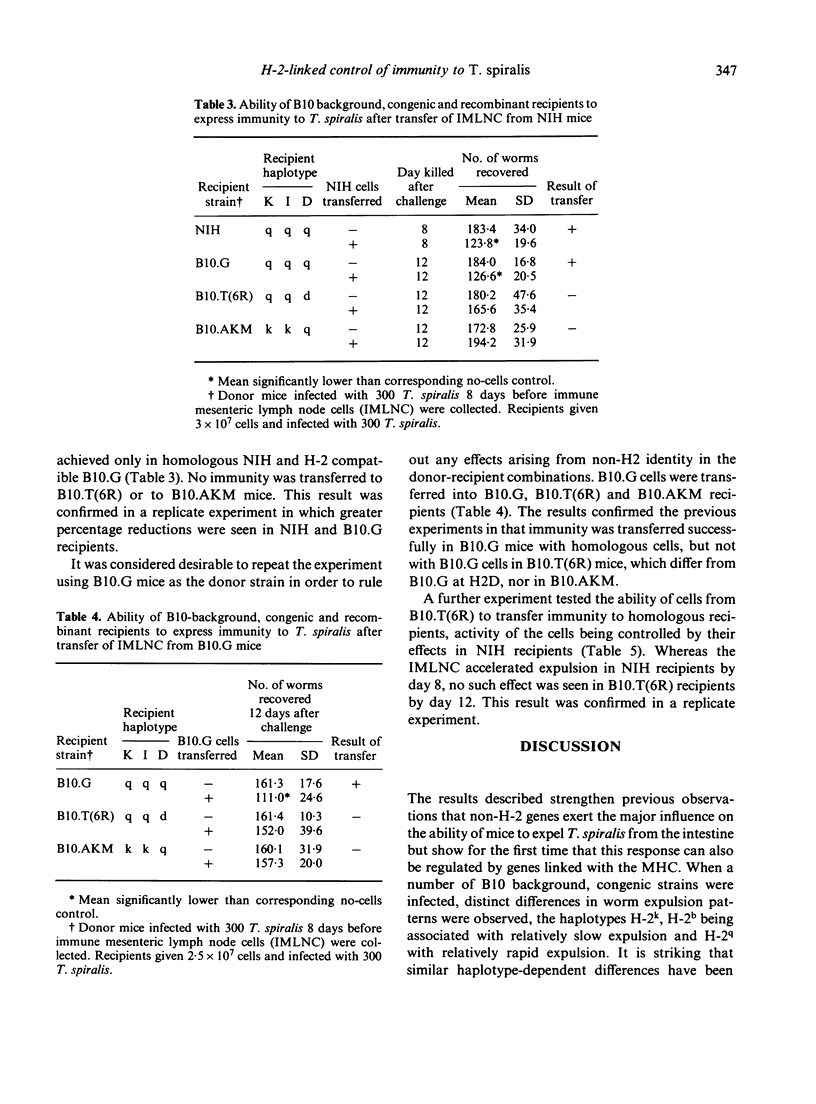
The time course of expulsion of adult Trichinella spiralis from the intestine was determined in B10 background, H-2 congenic and recombinant mice. Non-H-2 genes exerted the major influence on worm expulsion (i.e. determined rapid or slow response phenotype) but marked time course differences were seen among the slow responder B10 background strains, implying that H-2-linked genes also influence this parameter of immunity. Independent H-2q haplotype mice showed the most rapid expulsion, H-2k and H-2b the slowest. Data from H-2 recombinant mice carrying the q allele suggested that alleles at H-2K loci have a strong influence in immunity, but showed also that H-2D alleles exert a significant modulating effect. The q allele in otherwise susceptible k haplotype mice (B10.AKM) gave increased resistance; the d allele at H-2D in mice carrying the q resistance allele elsewhere [B10.T(6R)] gave decreased resistance. Adoptive transfers using immune mesenteric node lymphocytes (IMLNC) from a series of donors were used to identify how the modulating influence of H-2Dd was expressed in B10.T(6R) mice. IMLNC from this strain transferred immunity to recipients of other (histocompatible) strains, but IMLNC from such strains failed to accelerate expulsion in B10.T(6R) recipients as did homologous B10.T(6R) cells. Two alternative models are proposed to explain these results: either that H-2Dd influences the response of myeloid precursors to lymphocyte-derived factors, and thus the generation of intestinal inflammatory changes necessary for expulsion, or, on the assumption that the generation of intestinal inflammation requires initial cooperation between helper and effector lymphocytes, that H-2Dd is associated with a restricted ability of effector cells to respond to the helpers present in IMLNC.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alizadeh H., Wakelin D. Genetic factors controlling the intestinal mast cell response in mice infected with Trichinella spiralis. Clin Exp Immunol. 1982 Aug;49(2):331–337. [PMC free article] [PubMed] [Google Scholar]
- Hansen T. H., Ozato K., Melino M. R., Coligan J. E., Kindt T. J., Jandinski J. J., Sachs D. H. Immunochemical evidence in two haplotypes for at least three D region-encoded molecules, D, L, and R. J Immunol. 1981 May;126(5):1713–1716. [PubMed] [Google Scholar]
- Kong Y., David C. S., Giraldo A. A., Elrehewy M., Rose N. R. Regulation of autoimmune response to mouse thyroglobulin: influence of H-2D-end genes. J Immunol. 1979 Jul;123(1):15–18. [PubMed] [Google Scholar]
- Krco C. J., David C. S. Genetics of immune response: a survey. Crit Rev Immunol. 1981 Jan;1(3):211–257. [PubMed] [Google Scholar]
- Krco C. J., David C. S., Wassom D. L. Characterization of an in vitro proliferation response to solubilized Trichinella spiralis antigens: role of la antigens and Ly-1+ T cells. Cell Immunol. 1982 Apr;68(2):359–367. doi: 10.1016/0008-8749(82)90120-4. [DOI] [PubMed] [Google Scholar]
- Meruelo D., Leiberman M., Ginzton N., Deak B., McDevitt H. O. Genetic control of radiation leukemia virus-induced tumorigenesis. I. Role of the major murine histocompatibility complex, H-2. J Exp Med. 1977 Oct 1;146(4):1079–1087. doi: 10.1084/jem.146.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskimen J. A., Guertin D. P., Fan D. P., David C. S. Influence of H-2-linked genes on T cell proliferative and cytolytic responses to peptides of Sendai viral proteins. J Immunol. 1982 Apr;128(4):1522–1528. [PubMed] [Google Scholar]
- Strassmann G., Eshhar Z., Mozes E. Genetic regulation of delayed-type hypersensitivity responses to poly(LTyr,LGlu)-poly(DLAla)--poly(LLys). II. Evidence for a T-T-cell collaboration in delayed-type hypersensitivity responses and for a T-cell defect at the efferent phase in nonresponder H-2k mice. J Exp Med. 1980 Mar 1;151(3):628–636. doi: 10.1084/jem.151.3.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelin D., Donachie A. M. Genetic control of immunity to Trichinella spiralis. Donor bone marrow cells determine responses to infection in mouse radiation chimaeras. Immunology. 1981 Aug;43(4):787–792. [PMC free article] [PubMed] [Google Scholar]
- Wakelin D., Donachie A. M. Genetic control of immunity to parasites: adoptive transfer of immunity between inbred strains of mice characterized by rapid and slow immune expulsion of Trichinella spiralis. Parasite Immunol. 1980 Winter;2(4):249–260. doi: 10.1111/j.1365-3024.1980.tb00057.x. [DOI] [PubMed] [Google Scholar]
- Wakelin D. Genetic control of susceptibility and resistance to parasitic infection. Adv Parasitol. 1978;16:219–308. doi: 10.1016/s0065-308x(08)60575-8. [DOI] [PubMed] [Google Scholar]
- Wakelin D., Lloyd M. Immunity to primary and challenge infections of Trichinella spiralis in mice: a re-examination of conventional parameters. Parasitology. 1976 Apr;72(2):173–182. doi: 10.1017/s0031182000048472. [DOI] [PubMed] [Google Scholar]
- Wakelin D., Wilson M. M. Transfer of immunity to Trichinella spiralis in the mouse with mesenteric lymph node cells: time of appearance of effective cells in donors and expression of immunity in recipients. Parasitology. 1977 Jun;74(3):215–224. doi: 10.1017/s0031182000047843. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M. Restriction by H-2 gene complex of transfer of cell-mediated immunity to Listeria monocytogenes. Nature. 1974 Sep 20;251(5472):230–233. doi: 10.1038/251230a0. [DOI] [PubMed] [Google Scholar]


