Abstract
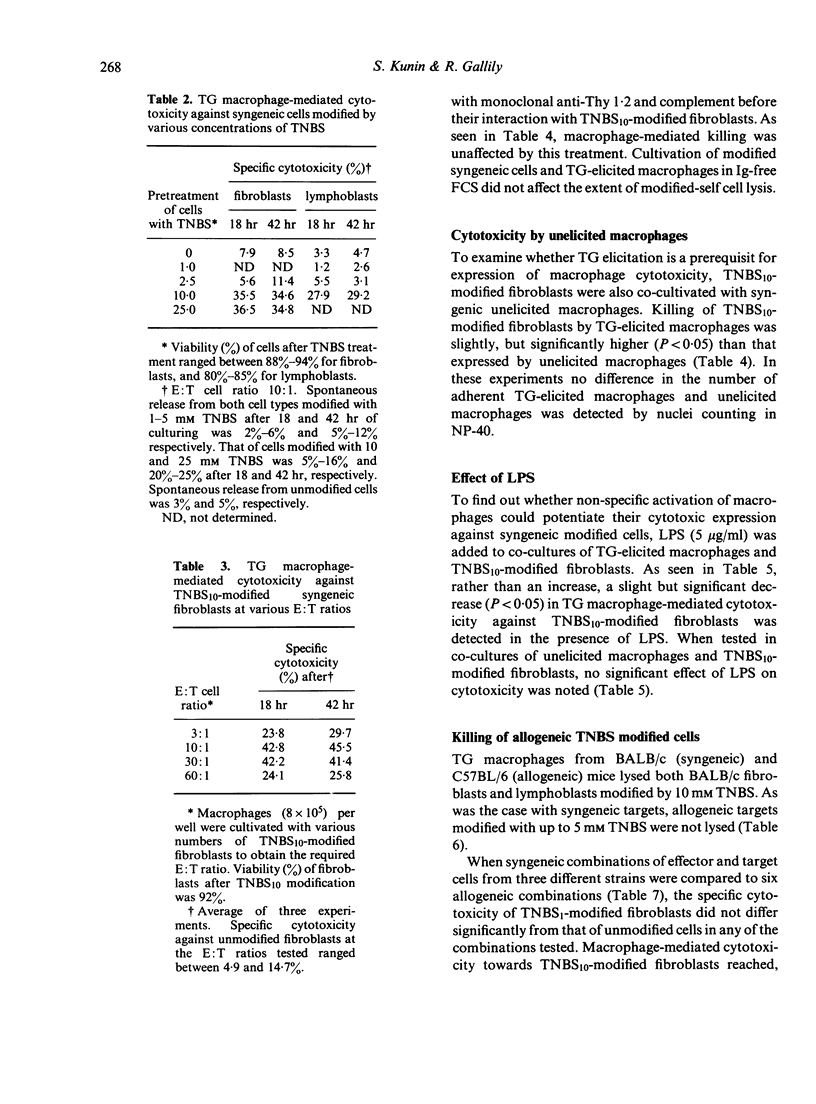
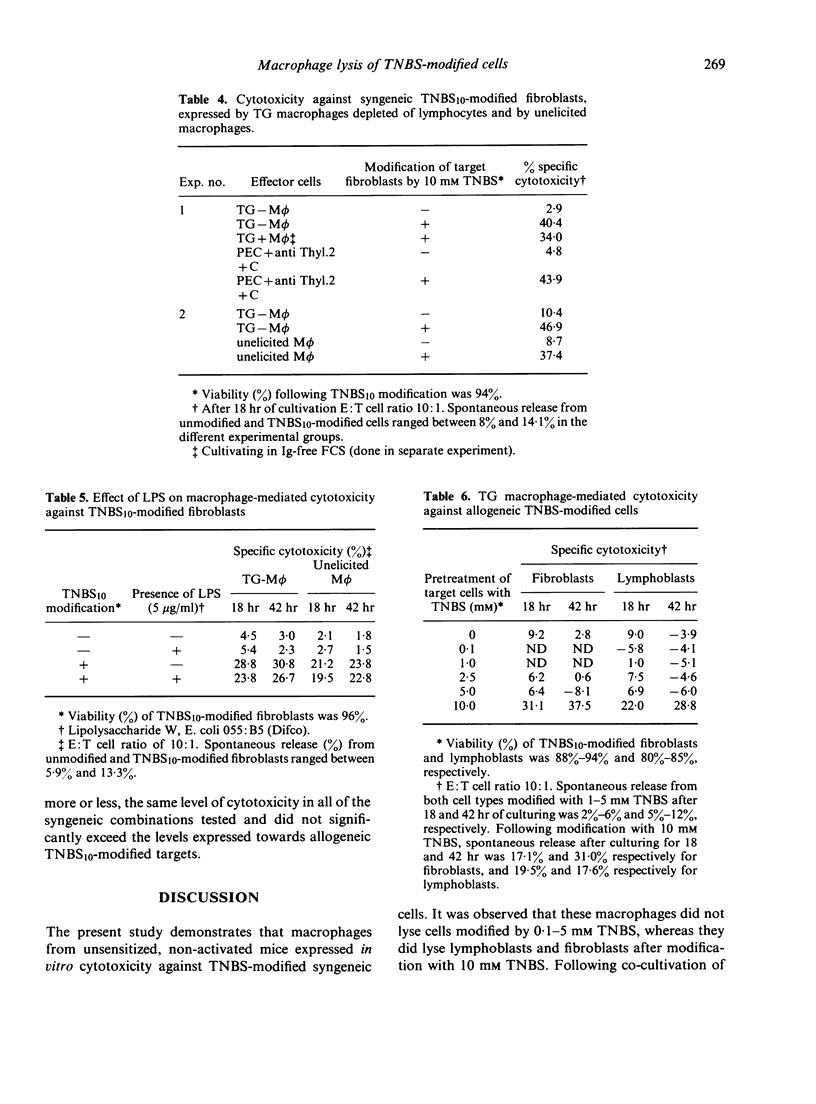
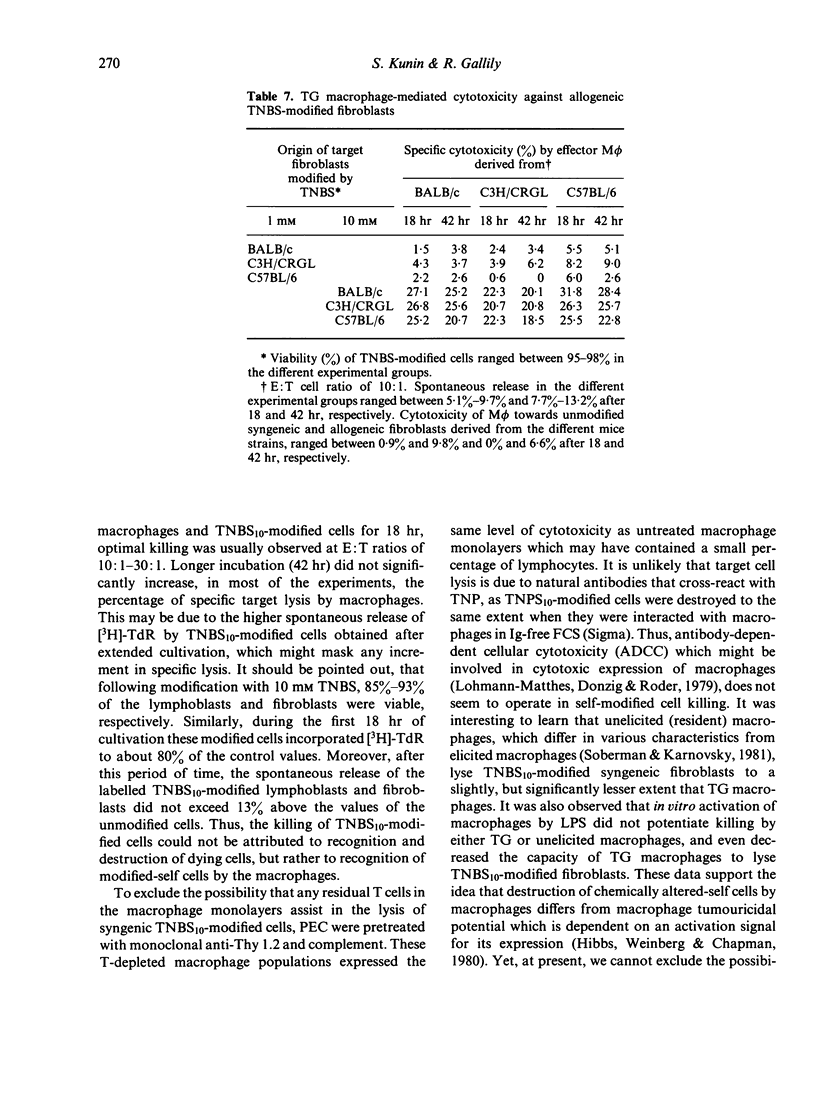
Peritoneal exudate macrophages from normal, untreated or thioglycollate-elicited mice, lysed syngeneic fibroblasts and lymphoblasts modified by 2,4,6-trinitrobenzene sulphonic acid (TNBS) in vitro. Optimal lysis of the hapten-modified cells by elicited macrophages was usually seen after 18 hr of co-cultivation at E:T ratios of 10:1-30:1. Cytotoxicity was expressed by macrophages depleted of T cells, and was not potentiated by LPS. Allogeneic TNBS-modified cells were lysed by non-immune, non-activated macrophages to the same extent as syngeneic modified targets, indicating that genetic restriction does not appear to play a role in macrophage-mediated cytolysis of TNBS-modified cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billings P., Burakoff S. J., Dorf M. E., Benacerraf B. Genetic control of cytolytic T-lymphocyte responses. I. Ir gene control of the specificity of cytolytic T-lymphocyte responses to trinitrophenyl-modified syngeneic cells. J Exp Med. 1978 Aug 1;148(2):341–350. doi: 10.1084/jem.148.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burakoff S. J., Germain R. N., Benacerraf B. Cross-reactive lysis of trinitrophenyl (TNP)-derivatized H-2 incompatible target cells by cytolytic T lymphocytes generated against syngeneic TNP spleen cells. J Exp Med. 1976 Dec 1;144(6):1609–1620. doi: 10.1084/jem.144.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabilly S., Gallily R. Non-immunological recognition and killing of xenogeneic cells by macrophages. I. Repertoire of recognition. Immunology. 1981 Oct;44(2):347–355. [PMC free article] [PubMed] [Google Scholar]
- Cabilly S., Gallily R. Non-immunological recognition and killing of xenogeneic cells by macrophages. II. Mechanism of killing. Immunology. 1981 Oct;44(2):357–365. [PMC free article] [PubMed] [Google Scholar]
- Ciavarra R., Forman J. Cell membrane antigens recognized by anti-viral and anti-trinitrophenyl cytotoxic T lymphocytes. Immunol Rev. 1981;58:73–94. doi: 10.1111/j.1600-065x.1981.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Forman J. On the role of the H-2 histocompatibility complex in determining the specificity of cytotoxic effector cells sensitized against syngeneic trinitrophenyl-modified targets. J Exp Med. 1975 Aug 1;142(2):403–418. doi: 10.1084/jem.142.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilheany P., Arora P. K., Levy R. B., Shearer G. M. H-2-linked genetic control of murine cell-mediated lympholysis to autologous cells modified with high and low concentrations of fluorescein isothiocyanate. Cell Immunol. 1981 Mar 15;59(1):97–105. doi: 10.1016/0008-8749(81)90437-8. [DOI] [PubMed] [Google Scholar]
- Lake P., Clark E. A., Khorshidi M., Sunshine G. H. Production and characterization of cytotoxic Thy-1 antibody-secreting hybrid cell lines. Detection of T cell subsets. Eur J Immunol. 1979 Nov;9(11):875–886. doi: 10.1002/eji.1830091109. [DOI] [PubMed] [Google Scholar]
- Levy R. B., Henkart P. A., Shearer G. M. Cell-mediated lympholysis responses against autologous cells modified with haptenic sulfhydryl reagents. II. Analysis of the genetic control and H-2-restricted pattern of cytotoxic responses to sulfhydryl and amino reactive reagents. J Immunol. 1981 Aug;127(2):529–534. [PubMed] [Google Scholar]
- Levy R. B., Shearer G. M. Regulation of T-cell-mediated lympholysis by the murine major histocompatibility complex. I. Preferential in vitro responses to trinitrophenyl-modified self K- and D-coded gene products in parental and F1 hybrid mouse strains. J Exp Med. 1979 Jun 1;149(6):1379–1392. doi: 10.1084/jem.149.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. B., Shearer G. M., Richardson J. C., Henkart P. A. Cell-mediated lympholytic responses against autologous cells modified with haptenic sulfhydryl reagents. I. Effector cells can recognize two distinct classes of hapten-reactive self sites on cell surface proteins. J Immunol. 1981 Aug;127(2):523–528. [PubMed] [Google Scholar]
- Lohmann-Matthes M. L., Domzig W., Roder J. Promonocytes have the functional characteristics of natural killer cells. J Immunol. 1979 Oct;123(4):1883–1886. [PubMed] [Google Scholar]
- Pohlit H., Haas W., von Boehmer H. Cytotoxic T cell responses to haptenated cells. I. Primary, secondary and long-term cultures. Eur J Immunol. 1979 Sep;9(9):681–690. doi: 10.1002/eji.1830090905. [DOI] [PubMed] [Google Scholar]
- Rehn T. G., Shearer G. M., Koren H. S., Inman J. K. Cell-mediated lympholysis of N-(3-nitro-4-hydroxy-5-iodophenylacetyl)-beta-anaylglycylglycyl-modified autologous lymphocytes. Effector cell specificity to modified cell surface components controlled by the H-2K and H-2D serological regions of the murine major histocompatibility complex. J Exp Med. 1976 Jan 1;143(1):127–142. doi: 10.1084/jem.143.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Verhulst A. M., Shearer G. M. Bifunctional major histocompatibility-linked genetic regulation of cell-mediated lympholysis to trinitrophenyl-modified autologous lymphocytes. J Exp Med. 1975 Oct 1;142(4):914–927. doi: 10.1084/jem.142.4.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroit A. J., Gallily R. Quantitative in vitro phagocytic rate measurements. J Immunol Methods. 1977;17(1-2):123–130. doi: 10.1016/0022-1759(77)90083-7. [DOI] [PubMed] [Google Scholar]
- Shearer G. M. Cell-mediated cytotoxicity to trinitrophenyl-modified syngeneic lymphocytes. Eur J Immunol. 1974 Aug;4(8):527–533. doi: 10.1002/eji.1830040802. [DOI] [PubMed] [Google Scholar]
- Shearer G. M., Schmitt-Verhulst A. M., Pettinelli C. B., Miller M. W., Gilheany P. E. H-2-linked genetic control of murine T-cell-mediated lympholysis to autologous cells modified with low concentrations of trinitrobenzene sulfonate. J Exp Med. 1979 Jun 1;149(6):1407–1423. doi: 10.1084/jem.149.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A., Burakoff S. J., Benacerraf B. The induction of cytolytic T lymphocytes with specificity for p-azophenylarsonate coupled syngeneic cells. J Immunol. 1978 Oct;121(4):1432–1436. [PubMed] [Google Scholar]
- Sugimoto M., Egashira Y., Pierres A., Greene M. I. Species restriction in the ability of TNP-derivatized cells to induce delayed hypersensitivity response. Cell Immunol. 1980 Sep 15;55(1):74–84. doi: 10.1016/0008-8749(80)90138-0. [DOI] [PubMed] [Google Scholar]
- Teh H. S., Phillips R. A., Miller R. G. Quantitative studies on the precursors of cytotoxic lymphocytes. V. The cellular basis for the cross-reactivity of TNP-specific clones. J Immunol. 1978 Nov;121(5):1711–1717. [PubMed] [Google Scholar]


