Abstract
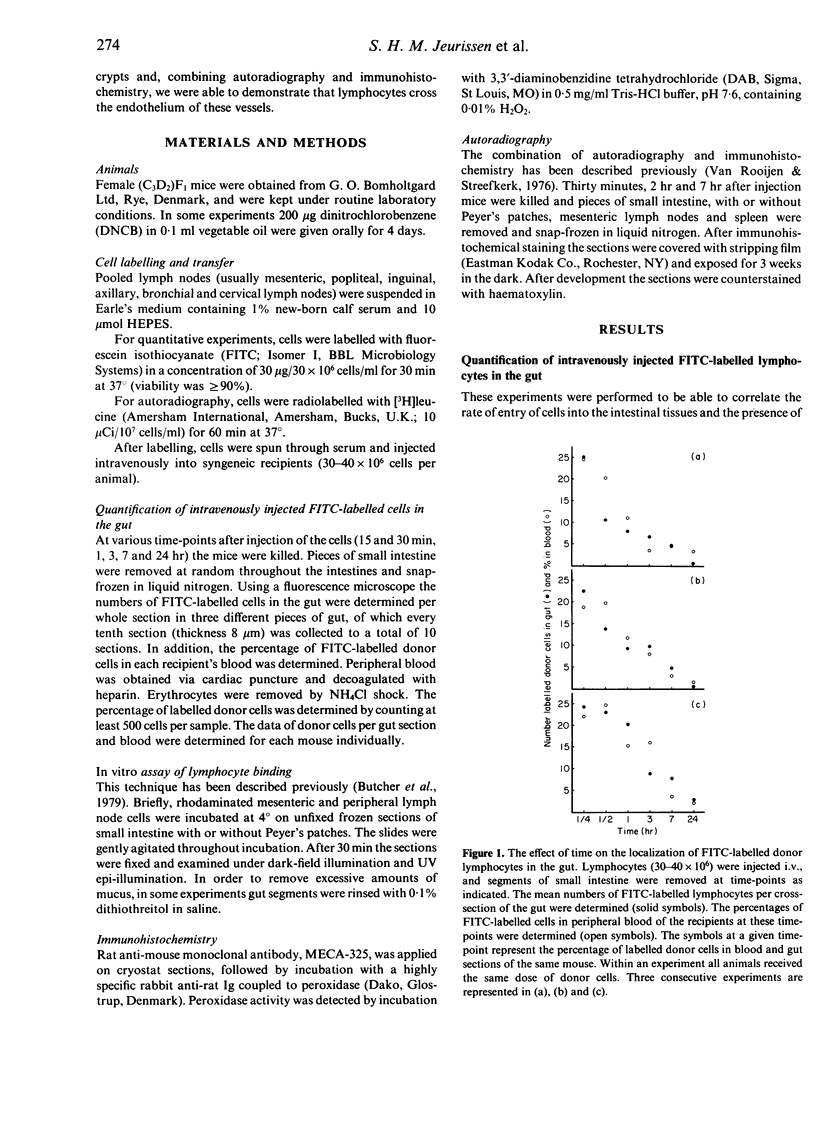
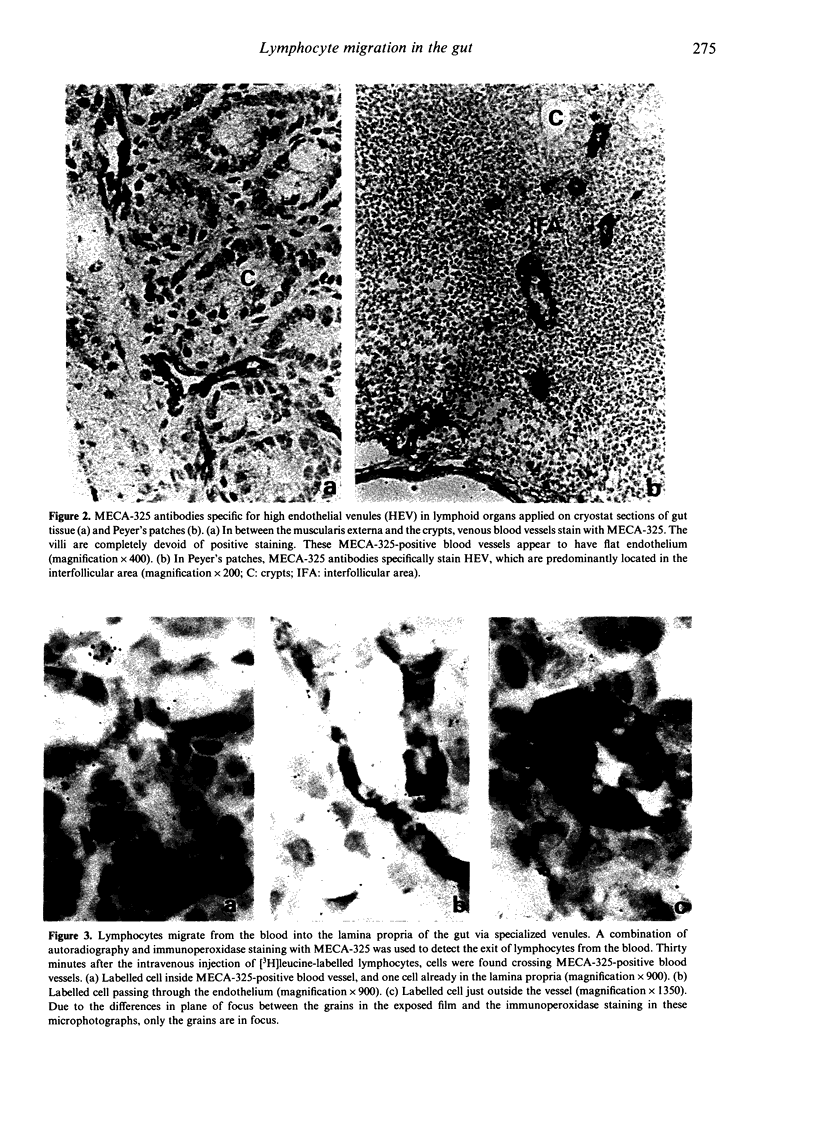
Migration of lymphocytes into the lamina propria of the small intestines was studied in mice using short-term in vivo migration experiments in combination with immunocytochemistry and autoradiography. The results show that, shortly after intravenous injection, most of the lymphocytes present in the lamina propria are actually located within the capillary network of the villi. Furthermore, it was shown that lymphocytes leave the blood stream and enter the lamina propria via small blood vessels at the base of the villi. These blood vessels can be discriminated by their positive staining with MECA-325, a monoclonal antibody that is specific for high endothelial venules (HEV) in lymphoid organs. From the results it is concluded that the gut contains specialized venules at specific sites, involved in the emigration of lymphocytes, comparable to HEV in lymphoid organs. The flatness of the endothelium of these MECA-325-positive intestinal blood vessels, which is in contrast to the situation in lymphoid organs, could not be changed by inducing an intestinal inflammation. This flatness may be directly correlated to the less efficient transmigration of lymphocytes, as demonstrated in our experiments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butcher E. C., Scollay R. G., Weissman I. L. Lymphocyte adherence to high endothelial venules: characterization of a modified in vitro assay, and examination of the binding of syngeneic and allogeneic lymphocyte populations. J Immunol. 1979 Nov;123(5):1996–2003. [PubMed] [Google Scholar]
- Butcher E. C., Scollay R. G., Weissman I. L. Organ specificity of lymphocyte migration: mediation by highly selective lymphocyte interaction with organ-specific determinants on high endothelial venules. Eur J Immunol. 1980 Jul;10(7):556–561. doi: 10.1002/eji.1830100713. [DOI] [PubMed] [Google Scholar]
- Cahill R. N., Poskitt D. C., Frost D. C., Trnka Z. Two distinct pools of recirculating T lymphocytes: migratory characteristics of nodal and intestinal T lymphocytes. J Exp Med. 1977 Feb 1;145(2):420–428. doi: 10.1084/jem.145.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin Y. H., Rasmussen R. A., Woodruff J. J., Easton T. G. A monoclonal anti-HEBFPP antibody with specificity for lymphocyte surface molecules mediating adhesion to Peyer's patch high endothelium of the rat. J Immunol. 1986 Apr 1;136(7):2556–2561. [PubMed] [Google Scholar]
- Duijvestijn A. M., Kerkhove M., Bargatze R. F., Butcher E. C. Lymphoid tissue- and inflammation-specific endothelial cell differentiation defined by monoclonal antibodies. J Immunol. 1987 Feb 1;138(3):713–719. [PubMed] [Google Scholar]
- Freemont A. J., Ford W. L. Functional and morphological changes in post-capillary venules in relation to lymphocytic infiltration into BCG-induced granulomata in rat skin. J Pathol. 1985 Sep;147(1):1–12. doi: 10.1002/path.1711470102. [DOI] [PubMed] [Google Scholar]
- Griscelli C., Vassalli P., McCluskey R. T. The distribution of large dividing lymph node cells in syngeneic recipient rats after intravenous injection. J Exp Med. 1969 Dec 1;130(6):1427–1451. doi: 10.1084/jem.130.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The gut-associated lymphoid system: nature and properties of the large dividing cells. Eur J Immunol. 1974 Jun;4(6):435–443. doi: 10.1002/eji.1830040610. [DOI] [PubMed] [Google Scholar]
- Hall J. G., Parry D. M., Smith M. E. The distribution and differentiation of lymph-borne immunoblasts after intravenous injection into syngeneic recipients. Cell Tissue Kinet. 1972 May;5(3):269–281. doi: 10.1111/j.1365-2184.1972.tb00365.x. [DOI] [PubMed] [Google Scholar]
- Hall J. G., Smith M. E. Homing of lymph-borne immunoblasts to the gut. Nature. 1970 Apr 18;226(5242):262–263. doi: 10.1038/226262a0. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Reichert R. A., Gallatin W. M., Bargatze R. F., Weissman I. L., Butcher E. C. Homing receptors and the control of lymphocyte migration. Immunol Rev. 1986 Jun;91:39–60. doi: 10.1111/j.1600-065x.1986.tb01483.x. [DOI] [PubMed] [Google Scholar]
- Jeurissen S. H., Sminia T., Kraal G. Selective emigration of suppressor T cells from Peyer's patches. Cell Immunol. 1984 Apr 15;85(1):264–269. doi: 10.1016/0008-8749(84)90297-1. [DOI] [PubMed] [Google Scholar]
- Kraal G., Duijvestijn A. M., Hendriks H. H. The endothelium of the high endothelial venule: a specialized endothelium with unique properties. Exp Cell Biol. 1987;55(1):1–10. doi: 10.1159/000163388. [DOI] [PubMed] [Google Scholar]
- Kraal G., Twisk A., Tan B., Scheper R. A surface molecule on guinea pig lymphocytes involved in adhesion and homing. Eur J Immunol. 1986 Dec;16(12):1515–1519. doi: 10.1002/eji.1830161208. [DOI] [PubMed] [Google Scholar]
- Marsh M. N. Studies of intestinal lymphoid tissue. I. Electron microscopic evidence of 'blast transformation' in epithelial lymphocytes of mouse small intestinal mucosa. Gut. 1975 Sep;16(9):665–674. doi: 10.1136/gut.16.9.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen R. A., Chin Y. H., Woodruff J. J., Easton T. G. Lymphocyte recognition of lymph node high endothelium. VII. Cell surface proteins involved in adhesion defined by monoclonal anti-HEBFLN (A.11) antibody. J Immunol. 1985 Jul;135(1):19–24. [PubMed] [Google Scholar]
- Scollay R. G., Butcher E. C., Weissman I. L. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980 Mar;10(3):210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- Smith J. B., McIntosh G. H., Morris B. The migration of cells through chronically inflamed tissues. J Pathol. 1970 Jan;100(1):21–29. doi: 10.1002/path.1711000104. [DOI] [PubMed] [Google Scholar]
- Stamper H. B., Jr, Woodruff J. J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976 Sep 1;144(3):828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng J. Transfer of lymphocytes of Peyer's patches between immunoglobulin allotype congenic mice: repopulation of the IgA plasma cells in the gut lamina propria. J Immunol. 1981 Nov;127(5):2039–2043. [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., PHILLIPS-Quagliata J. M., Lamm M. E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979 Oct;123(4):1705–1708. [PubMed] [Google Scholar]
- van Rooijen N., Streefkerk J. G. Autoradiography and immunohistoperoxidase techniques applied to the same tissue section. J Immunol Methods. 1976;10(4):379–383. doi: 10.1016/0022-1759(76)90032-6. [DOI] [PubMed] [Google Scholar]
- van der Brugge-Gamelkoorn G. J., Kraal G. The specificity of the high endothelial venule in bronchus-associated lymphoid tissue (BALT). J Immunol. 1985 Jun;134(6):3746–3750. [PubMed] [Google Scholar]