Abstract
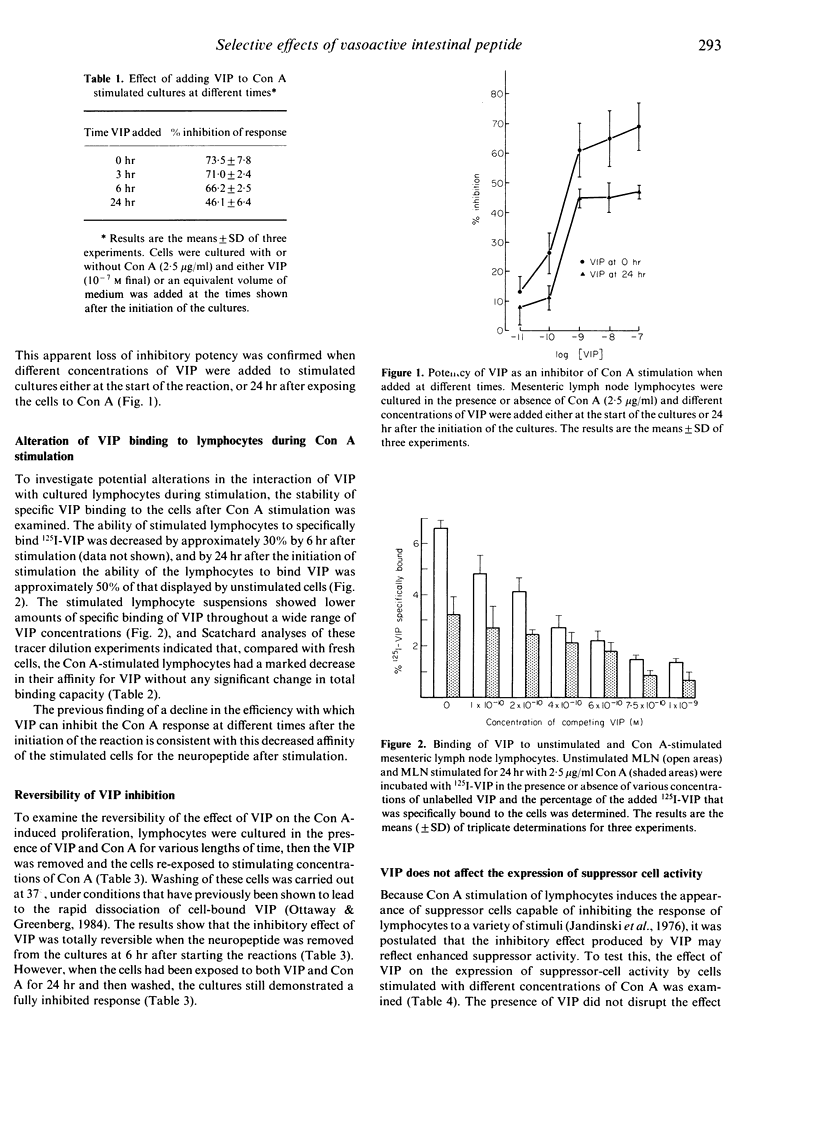
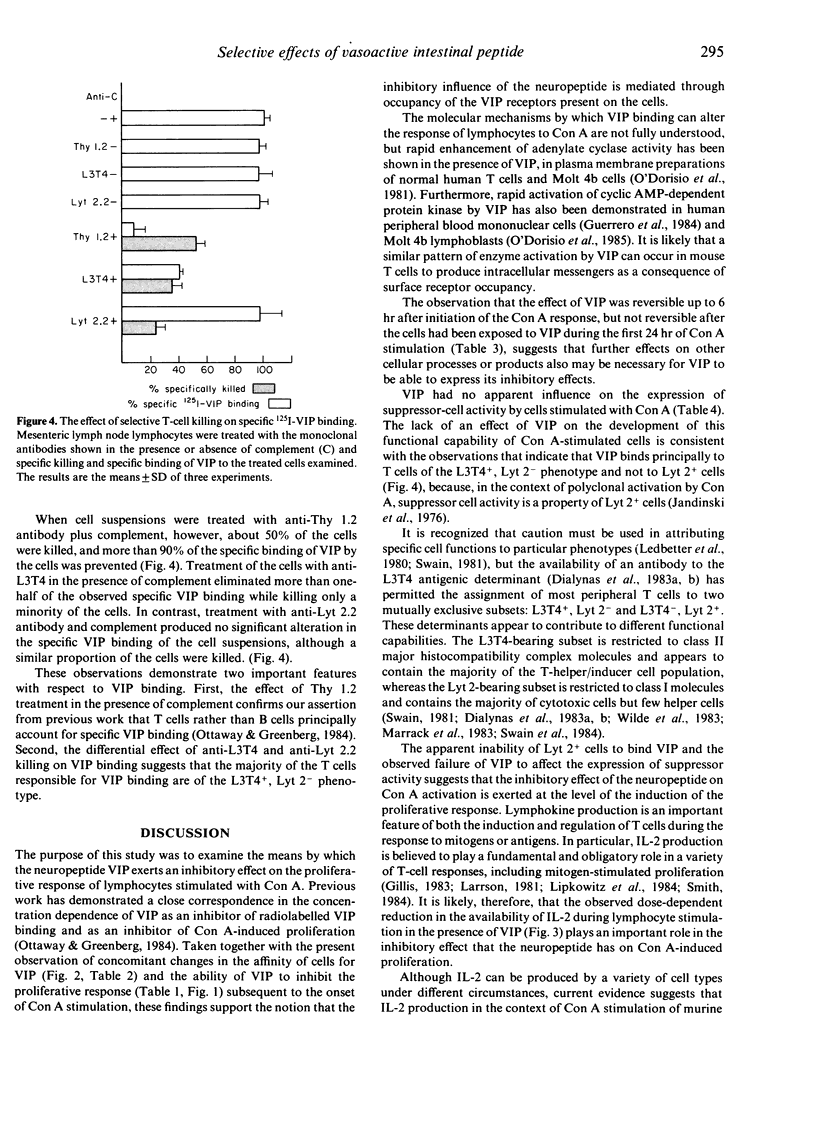
Murine lymphocytes have been shown previously to possess high-affinity specific receptors for the neuropeptide vasoactive intestinal peptide (VIP). This study examines the cellular basis for modulation of concanavalin A (Con A)-induced T-cell responses by this neuropeptide. VIP was most effective as an inhibitor when added at the initiation of the mitogen response. The loss of potency when VIP was added later in the response was accompanied by a decrease in the affinity of stimulated cells for the neuropeptide. The inhibitory influence of VIP was reversible if the neuropeptide was removed from stimulated cell cultures up to 6 hr after the initiation of stimulation. In contrast, VIP-mediated inhibition was fully developed once the stimulated cells had been exposed to the neuropeptide for 24 hr. The presence of VIP led to a decreased production of interleukin-2 (IL-2) by the stimulated lymphocytes, but did not affect the expression of Con A-induced suppressor cell activity by cultured lymphocytes. Studies of the effect of selective, complement-mediated killing of cells with Thy 1, L3T4 and Lyt 2 monoclonal antibodies showed that the majority of the VIP bound by the lymphocytes was accounted for by binding to L3T4+, Lyt 2- T cells. It was concluded that VIP exerts its influence over Con A-stimulated proliferation by selective regulation of T-cell subsets.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beed E. A., O'Dorisio M. S., O'Dorisio T. M., Gaginella T. S. Demonstration of a functional receptor for vasoactive intestinal polypeptide on Molt 4b T lymphoblasts. Regul Pept. 1983 Apr;6(1):1–12. doi: 10.1016/0167-0115(83)90129-5. [DOI] [PubMed] [Google Scholar]
- Danek A., O'Dorisio M. S., O'Dorisio T. M., George J. M. Specific binding sites for vasoactive intestinal polypeptide on nonadherent peripheral blood lymphocytes. J Immunol. 1983 Sep;131(3):1173–1177. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Gillis S. Interleukin 2: biology and biochemistry. J Clin Immunol. 1983 Jan;3(1):1–13. doi: 10.1007/BF00919133. [DOI] [PubMed] [Google Scholar]
- Guerrero J. M., Prieto J. C., Calvo J. R., Goberna R. Activation of cyclic AMP-dependent protein kinase by VIP in blood mononuclear cells. Peptides. 1984 Mar-Apr;5(2):371–373. doi: 10.1016/0196-9781(84)90236-5. [DOI] [PubMed] [Google Scholar]
- Guerrero J. M., Prieto J. C., Elorza F. L., Ramirez R., Goberna R. Interaction of vasoactive intestinal peptide with human blood mononuclear cells. Mol Cell Endocrinol. 1981 Feb;21(2):151–160. doi: 10.1016/0303-7207(81)90052-6. [DOI] [PubMed] [Google Scholar]
- Jandinski J., Cantor H., Tadakuma T., Peavy D. L., Pierce C. W. Separation of helper T cells from suppressor T cells expressing different Ly components. I. Polyclonal activation: suppressor and helper activities are inherent properties of distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1382–1390. doi: 10.1084/jem.143.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson E. L. Mechanism of T cell activation. II. Antigen- and lectin-dependent acquisition of responsiveness to TCGF is a nonmitogenic, active response of resting T cells. J Immunol. 1981 Apr;126(4):1323–1326. [PubMed] [Google Scholar]
- Larsson L. I., Polak J. M., Buffa R., Sundler F., Solcia E. On the immunocytochemical localization of the vasoactive intestinal polypeptide. J Histochem Cytochem. 1979 May;27(5):936–938. doi: 10.1177/27.5.479555. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkowitz S., Greene W. C., Rubin A. L., Novogrodsky A., Stenzel K. H. Expression of receptors for interleukin 2: Role in the commitment of T lymphocytes to proliferate. J Immunol. 1984 Jan;132(1):31–37. [PubMed] [Google Scholar]
- Malek T. R., Schmidt J. A., Shevach E. M. The murine IL 2 receptor. III. Cellular requirements for the induction of IL 2 receptor expression on T cell subpopulations. J Immunol. 1985 Apr;134(4):2405–2413. [PubMed] [Google Scholar]
- Marrack P., Endres R., Shimonkevitz R., Zlotnik A., Dialynas D., Fitch F., Kappler J. The major histocompatibility complex-restricted antigen receptor on T cells. II. Role of the L3T4 product. J Exp Med. 1983 Oct 1;158(4):1077–1091. doi: 10.1084/jem.158.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. A. IL 2 production by mitogen-stimulated T cell subsets: helper-precursors are predominantly Lyt-2-. J Immunol. 1983 Dec;131(6):2864–2867. [PubMed] [Google Scholar]
- O'Dorisio M. S., Hermina N. S., O'Dorisio T. M., Balcerzak S. P. Vasoactive intestinal polypeptide modulation of lymphocyte adenylate cyclase. J Immunol. 1981 Dec;127(6):2551–2554. [PubMed] [Google Scholar]
- O'Dorisio M. S., Wood C. L., Wenger G. D., Vassalo L. M. Cyclic AMP-dependent protein kinase in Molt 4b lymphoblasts: identification by photoaffinity labeling and activation in intact cells by vasoactive intestinal polypeptide (VIP) and peptide histidine isoleucine (PHI). J Immunol. 1985 Jun;134(6):4078–4086. [PubMed] [Google Scholar]
- Ottaway C. A., Bernaerts C., Chan B., Greenberg G. R. Specific binding of vasoactive intestinal peptide to human circulating mononuclear cells. Can J Physiol Pharmacol. 1983 Jul;61(7):664–671. doi: 10.1139/y83-103. [DOI] [PubMed] [Google Scholar]
- Ottaway C. A., Greenberg G. R. Interaction of vasoactive intestinal peptide with mouse lymphocytes: specific binding and the modulation of mitogen responses. J Immunol. 1984 Jan;132(1):417–423. [PubMed] [Google Scholar]
- Rich R. R., Rich S. S. Biological expressions of lymphocyte activation. IV. Concanavalin A-activated suppressor cells in mouse mixed lymphocyte reactions. J Immunol. 1975 Mar;114(3):1112–1115. [PubMed] [Google Scholar]
- Smith K. A. Interleukin 2. Annu Rev Immunol. 1984;2:319–333. doi: 10.1146/annurev.iy.02.040184.001535. [DOI] [PubMed] [Google Scholar]
- Stanisz A. M., Befus D., Bienenstock J. Differential effects of vasoactive intestinal peptide, substance P, and somatostatin on immunoglobulin synthesis and proliferations by lymphocytes from Peyer's patches, mesenteric lymph nodes, and spleen. J Immunol. 1986 Jan;136(1):152–156. [PubMed] [Google Scholar]
- Swain S. L., Dialynas D. P., Fitch F. W., English M. Monoclonal antibody to L3T4 blocks the function of T cells specific for class 2 major histocompatibility complex antigens. J Immunol. 1984 Mar;132(3):1118–1123. [PubMed] [Google Scholar]
- Swain S. L. Significance of Lyt phenotypes: Lyt2 antibodies block activities of T cells that recognize class 1 major histocompatibility complex antigens regardless of their function. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7101–7105. doi: 10.1073/pnas.78.11.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Trefts P. E., Tse H. Y., Dutton R. W. The significance of T-B collaboration across haplotype barriers. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):597–609. doi: 10.1101/sqb.1977.041.01.069. [DOI] [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]


