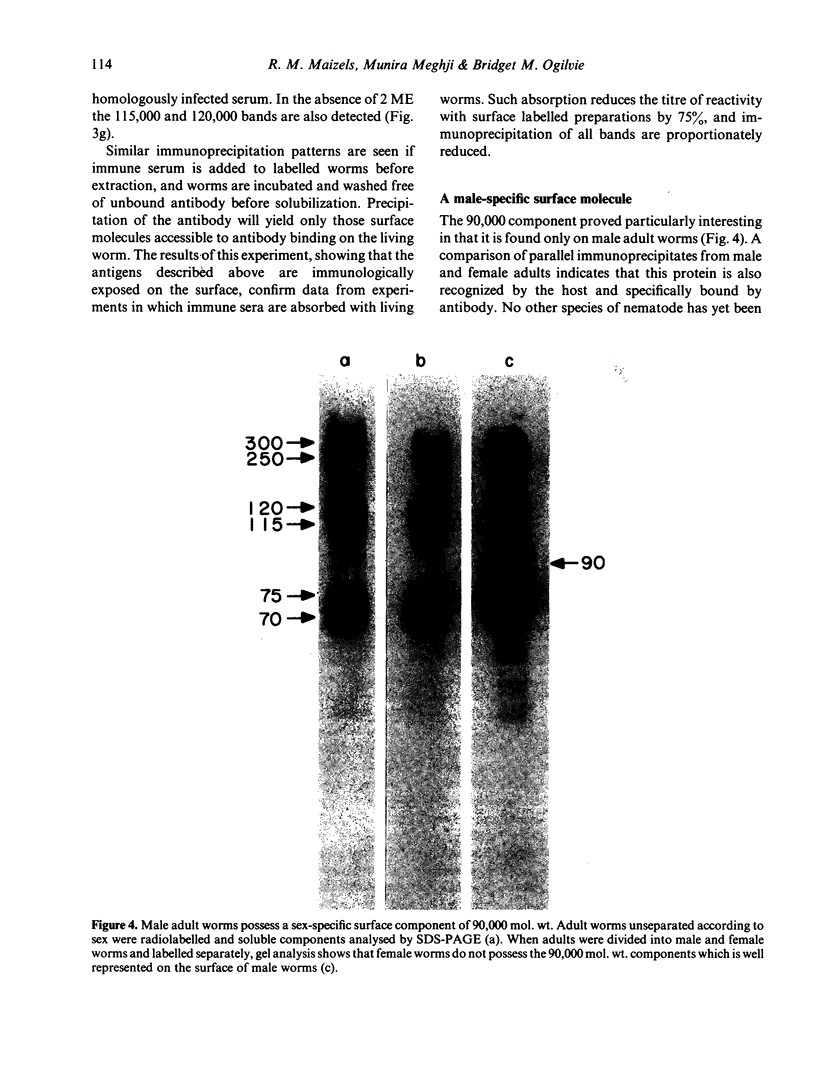
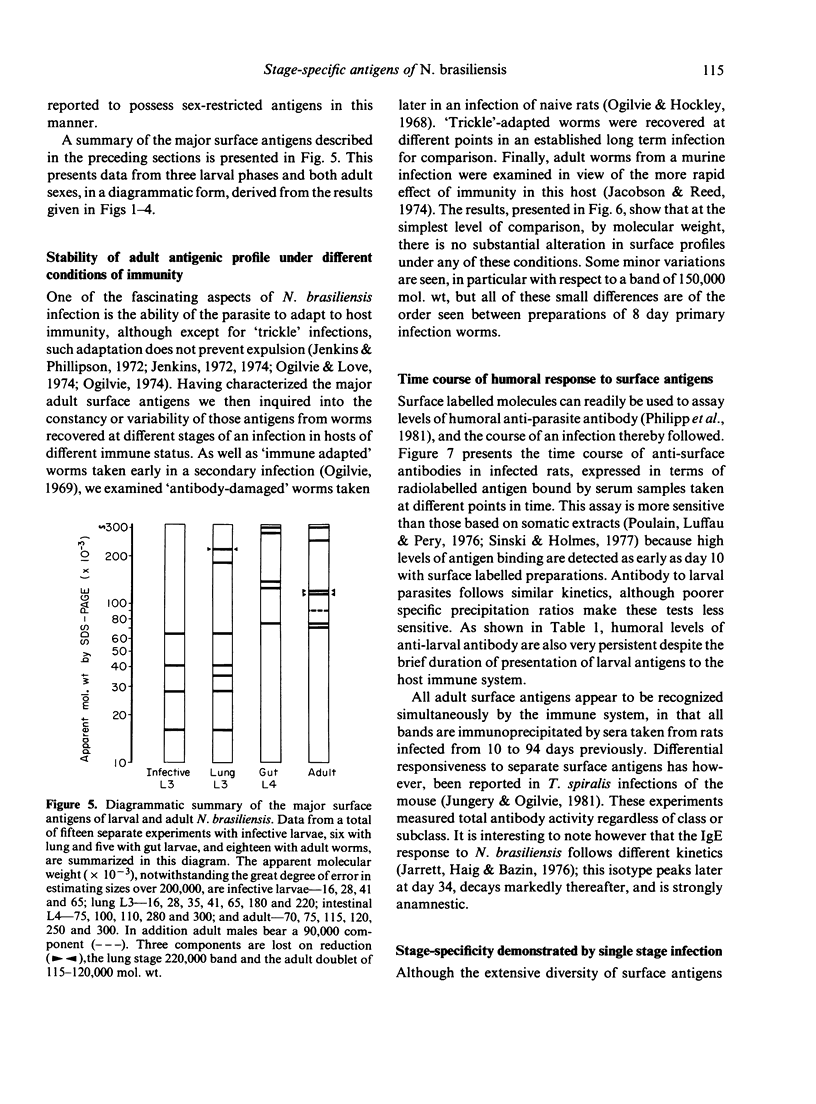
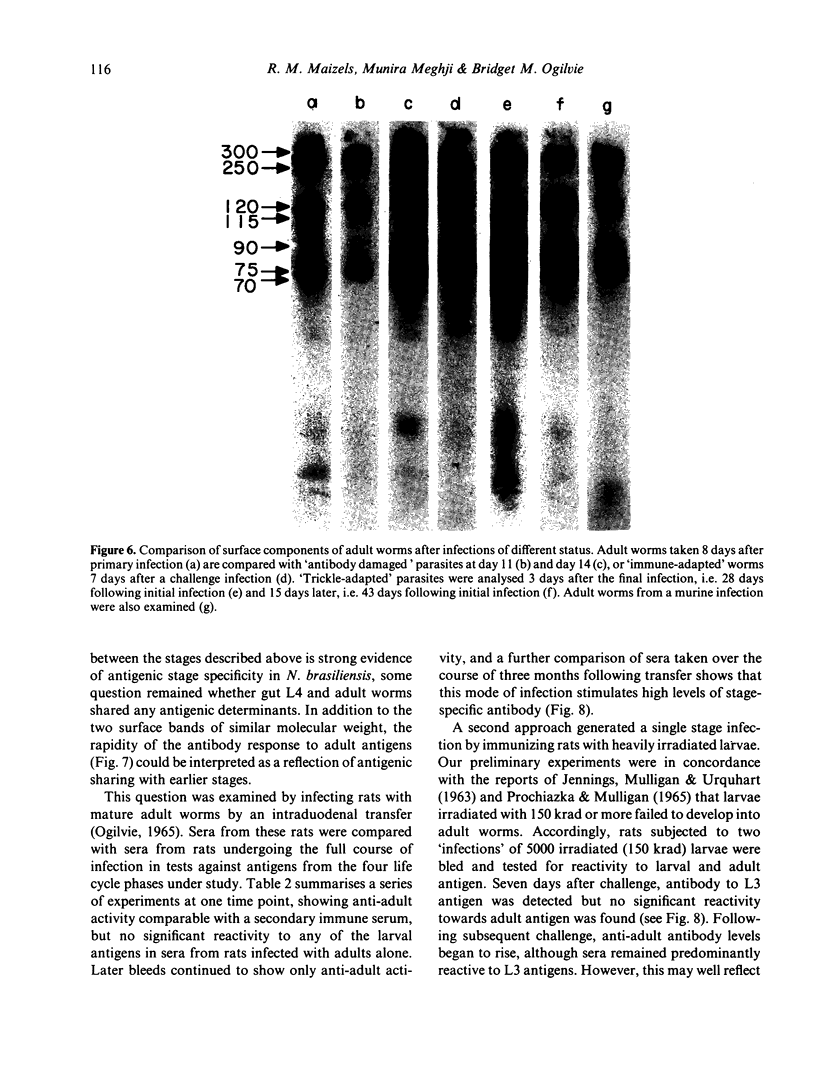
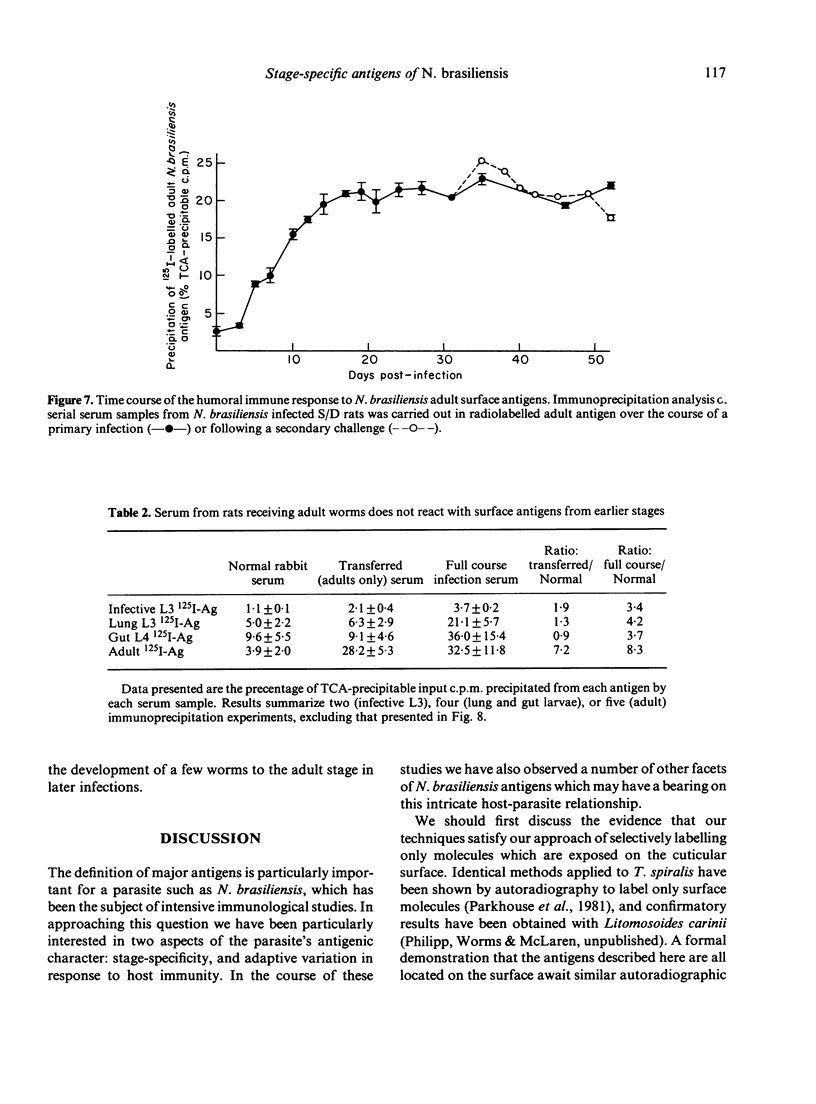
Abstract
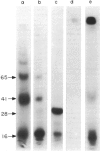
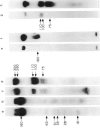
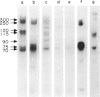
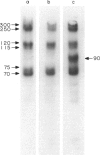
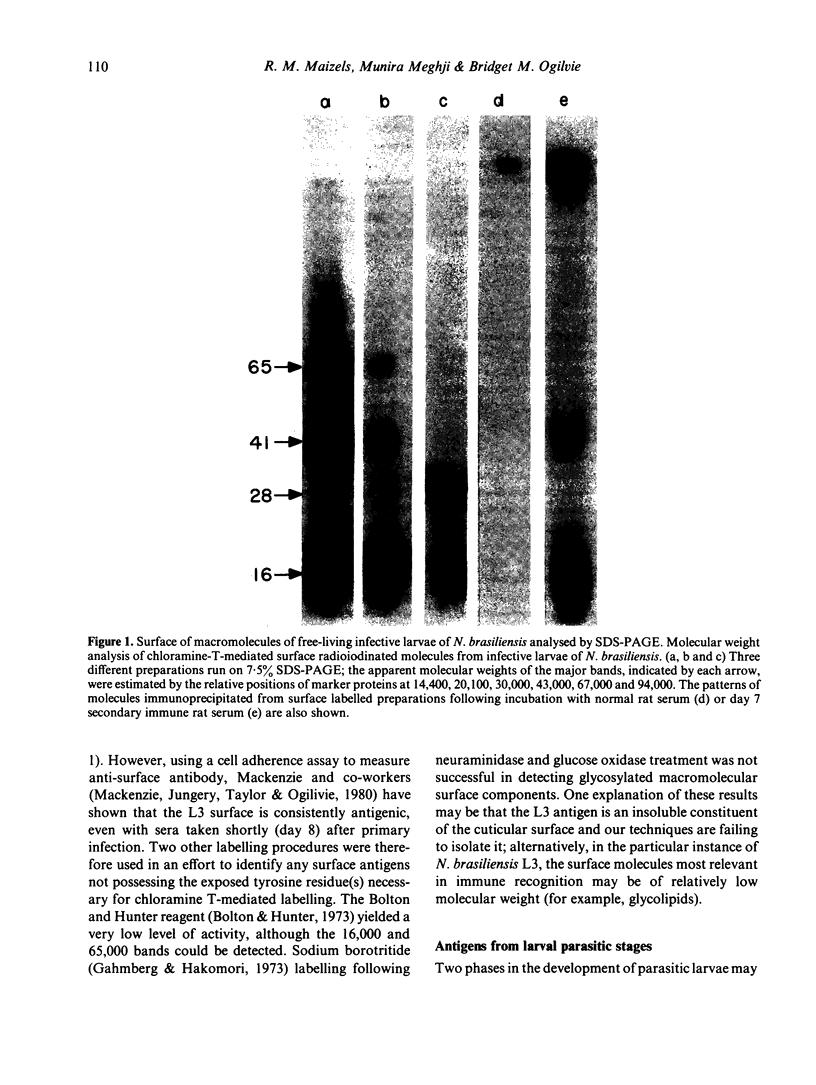
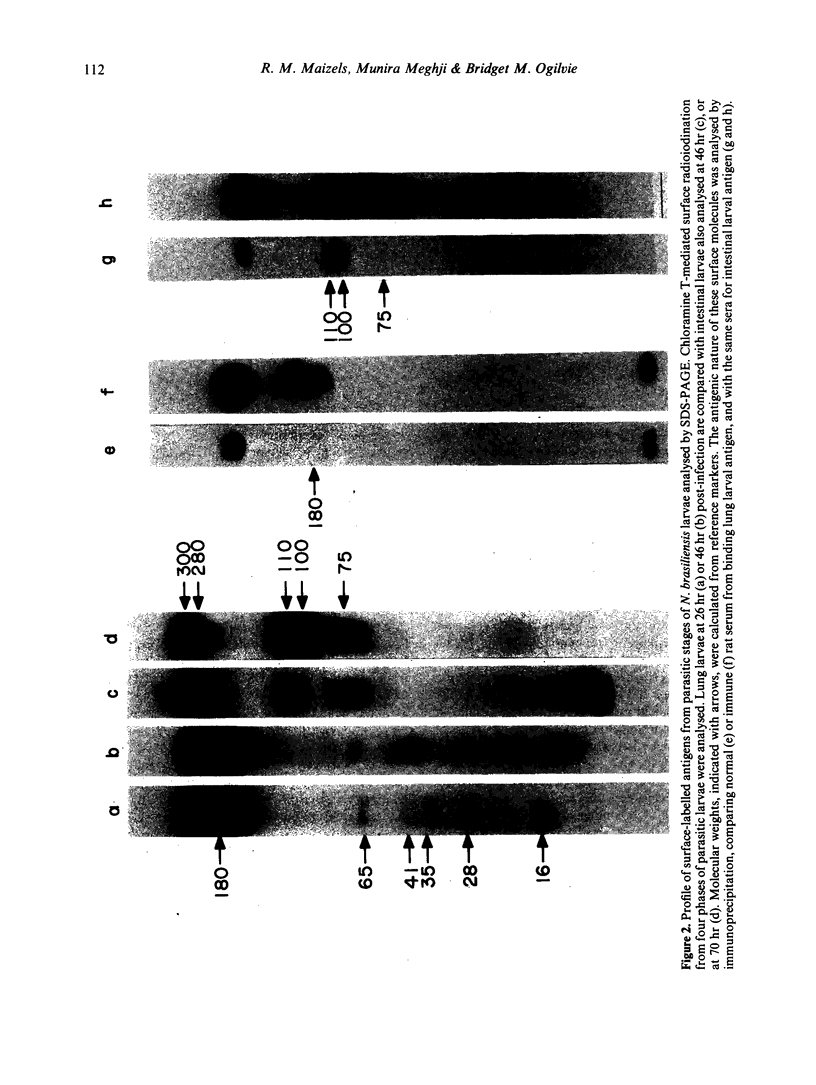
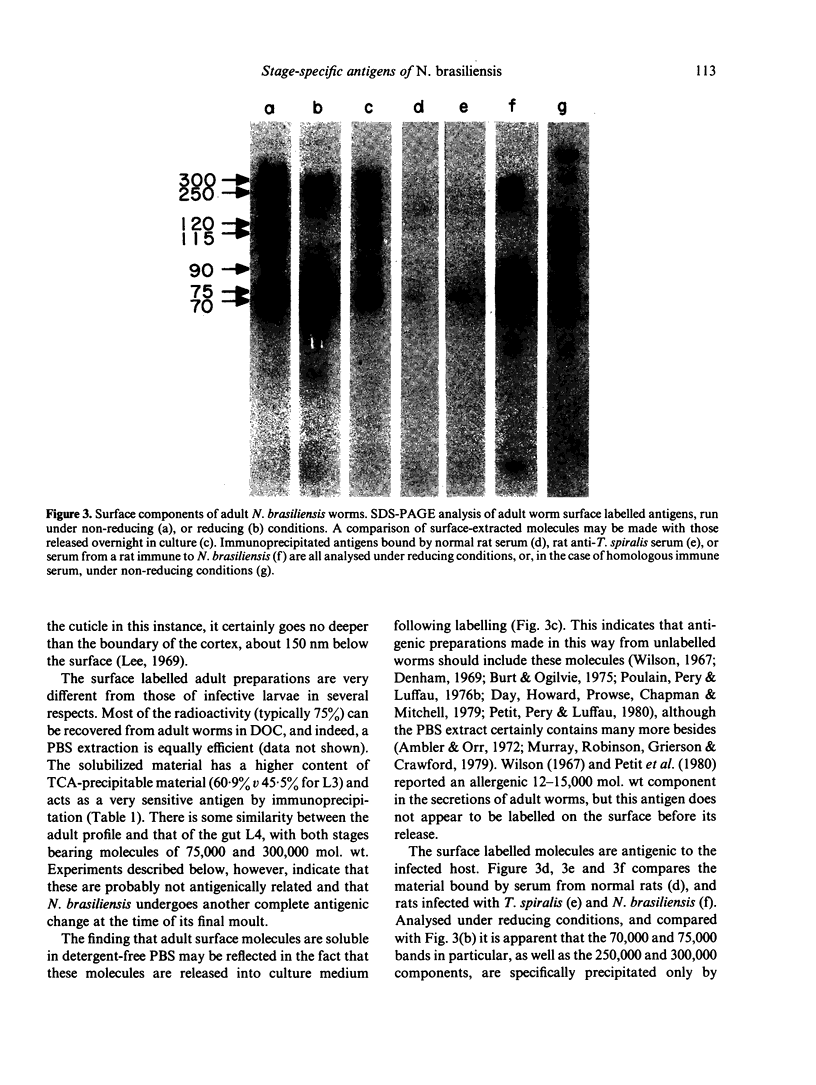
Surface molecules of parasitic stages of the nematode Nippostrongylus brasiliensis can be readily iodinated by the chloramine T technique, and assessed for antigenic reactivity with humoral antibody from infected animals. Free-living infective larvae are less amenable to analysis by this, or similar methods, but within 18 hr of larvae entering the host, new macromolecular surface antigens can be detected. The parasites change their surface antigens twice more in the course of the maturation to the adult stage. Surface antigens are stage-specific: lung larvae (L3), intestinal larvae (L4) and gut-living adults each possess characteristic sets of cuticular molecules. Single stage infections result in antibody reactive only to the antigens from the homologous stage. The adult surface appears to bear the greatest number of antigens, one of which is found only on the male worm. The composition of these antigens does not differ grossly between adult worms from a naive or immune host, or worms established after the adaptation of a 'trickle' (multiple low dose) infection. There appears to be an interesting contrast between the rapidity and extent of changes in surface antigens in the early phases of infection, and the stability of adult antigens analysed at different points in the host immune response.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler J., Orr T. S. Studies on an allergenic component extracted from N. brasiliensis. Immunochemistry. 1972 Mar;9(3):263–272. doi: 10.1016/0019-2791(72)90091-2. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt J. S., Ogilvie B. M. In vitro maintenance of nematode parasites assayed by acetylcholinesterase and allergen secretion. Exp Parasitol. 1975 Aug;38(1):75–82. doi: 10.1016/0014-4894(75)90039-9. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E., Sturrock R. F., Houba V., Mahmoud A. A., Sher A., Rees P. H. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature. 1975 Aug 28;256(5520):727–729. doi: 10.1038/256727a0. [DOI] [PubMed] [Google Scholar]
- Day K. P., Howard R. J., Prowse S. J., Chapman C. B., Mitchell G. F. Studies on chronic versus transient intestinal nematode infections in mice. I. A. comparison of responses to excretory/secretory (ES) products of Nippostrongylus brasiliensis and Nematospiroides dubius worms. Parasite Immunol. 1979 Autumn;1(3):217–239. doi: 10.1111/j.1365-3024.1979.tb00708.x. [DOI] [PubMed] [Google Scholar]
- Denham D. A. Secretion of metabolic antigen by nippostrongylus brasiliensis in vitro. J Parasitol. 1969 Jun;55(3):676–677. [PubMed] [Google Scholar]
- Edwards A. J., Burt J. S., Ogilvie B. M. The effect of immunity upon some enzymes of the parasitic nematode, Nippostrongylus brasiliensis. Parasitology. 1971 Apr;62(2):339–347. doi: 10.1017/s0031182000071572. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Jacobson R. H., Reed N. D., Manning D. D. Expulsion of Nippostrongylus brasiliensis from mice lacking antibody production potential. Immunology. 1977 Jun;32(6):867–874. [PMC free article] [PubMed] [Google Scholar]
- Jacobson R. H., Reed N. D. The immune response of congenitally athymic (nude) mice to the intestinal nematode Nippostrongylus brasiliensis. Proc Soc Exp Biol Med. 1974 Dec;147(3):667–670. doi: 10.3181/00379727-147-38412. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E., Haig D. M. Time course studies on rat IgE production in N. Brasiliensis infection. Clin Exp Immunol. 1976 May;24(2):346–351. [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Jarrett W. F., Urquhart G. M. Immunological unresponsiveness in adult rats to the nematode Nippostrongylus brasiliensis induced by infection in early life. Nature. 1966 Sep 17;211(5055):1310–1311. doi: 10.1038/2111310a0. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E., Jarrett W. F., Urquhart G. M. Quantitative studies on the kinetics of establishment and expulsion of intestinal nematode populations in susceptible and immune hosts. Nippostrongylus brasiliensis in the rat. Parasitology. 1968 Aug;58(3):625–639. doi: 10.1017/s0031182000028924. [DOI] [PubMed] [Google Scholar]
- Jenkins D. C. Nippostrongylus brasiliensis: observations on the comparative immunogenicity of adult worms from primary and immune-adapted infections. Parasitology. 1972 Dec;65(3):547–550. doi: 10.1017/s0031182000044152. [DOI] [PubMed] [Google Scholar]
- Jenkins D. C., Phillipson R. F. Evidence that the nematode Nippostrongylus brasiliensis can adapt to and overcome the effects of host immunity. Int J Parasitol. 1972 Sep;2(3):353–359. doi: 10.1016/0020-7519(72)90073-2. [DOI] [PubMed] [Google Scholar]
- Jones V. E., Ogilvie B. M. Protective immunity to Nippostrongylus brasiliensis: the sequence of events which expels worms from the rat intestine. Immunology. 1971 Apr;20(4):549–561. [PMC free article] [PubMed] [Google Scholar]
- Keller R., Keist R. Protective immunity to Nippostrongylus brasiliensis in the rat. Central role of the lymphocyte in worm expulsion. Immunology. 1972 May;22(5):767–773. [PMC free article] [PubMed] [Google Scholar]
- Kelly J. D., Dineen J. K. The cellular transfer to immunity to Nippostrongylus brasiliensis in inbred rats (Lewis strain). Immunology. 1972 Feb;22(2):199–210. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Love R. J., Ogilvie B. M. Nippostrongylus brasiliensis in young rats. Lymphocytes expel larval infections but not adult worms. Clin Exp Immunol. 1975 Jul;21(1):155–162. [PMC free article] [PubMed] [Google Scholar]
- MULLIGAN W., URQUHART G. M., JENNINGS F. W., NEILSON J. T. IMMUNOLOGICAL STUDIES ON NIPPOSTRONGYLUS BRASILIENSIS INFECTION IN THE RAT: "SELF-CURE" PHENOMENON. Exp Parasitol. 1965 Jun;16:341–347. doi: 10.1016/0014-4894(65)90056-1. [DOI] [PubMed] [Google Scholar]
- Mackenzie C. D., Jungery M., Taylor P. M., Ogilvie B. M. Activation of complement, the induction of antibodies to the surface of nematodes and the effect of these factors and cells on worm survival in vitro. Eur J Immunol. 1980 Aug;10(8):594–601. doi: 10.1002/eji.1830100805. [DOI] [PubMed] [Google Scholar]
- Mackenzie C. D., Preston P. M., Ogilvie B. M. Immunological properties of the surface of parasitic nematodes. Nature. 1978 Dec 21;276(5690):826–828. doi: 10.1038/276826a0. [DOI] [PubMed] [Google Scholar]
- Maizels R. M., Philipp M., Ogilvie B. M. Molecules on the surface of parasitic nematodes as probes of the immune response in infection. Immunol Rev. 1982;61:109–136. doi: 10.1111/j.1600-065x.1982.tb00375.x. [DOI] [PubMed] [Google Scholar]
- Miller H. R. Expulsion of Nippostrongylus brasiliensis from rats protected with serum. I. The efficacy of sera from singly and multiply infected donors related to time of administration and volume of serum injected. Immunology. 1980 Jul;40(3):325–334. [PMC free article] [PubMed] [Google Scholar]
- Murray M., Robinson P. B., Grierson C., Crawford R. A. Immunization against Nippostrongylus brasiliensis in the rat. A study on the use of antigen extracted from adult parasites and the parameters which influence the level of protection. Acta Trop. 1979 Dec;36(4):297–322. [PubMed] [Google Scholar]
- Nawa Y., Miller H. R. Protection against Nippostrongylus brasiliensis by adoptive immunization with immune thoracic duct lymphocytes. Cell Immunol. 1978 Apr;37(1):51–60. doi: 10.1016/0008-8749(78)90173-9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- OGILVIE B. M. ROLE OF ADULT WORMS IN IMMUNITY OF RATS TO NIPPOSTRONGYLUS BRASILIENSIS. Parasitology. 1965 May;55:325–335. doi: 10.1017/s0031182000068797. [DOI] [PubMed] [Google Scholar]
- Ogilvie B. M., Hockley D. J. Effects of immunity of Nippostrongylus brasiliensis adult worms: reversible and irreversible changes in infectivity, reproduction, and morphology. J Parasitol. 1968 Dec;54(6):1073–1084. [PubMed] [Google Scholar]
- Ogilvie B. M., Jones V. E. Parasitological review. Nippostrongylus brasiliensis: a review of immunity and host-parasite relationship in the rat. Exp Parasitol. 1971 Feb;29(1):138–177. doi: 10.1016/0014-4894(71)90021-x. [DOI] [PubMed] [Google Scholar]
- Ogilvie B. M., Jones V. E. Passive protection with cells or antiserum against Nippostronglylus brasiliensis in the rat. Parasitology. 1968 Nov;58(4):939–949. doi: 10.1017/s0031182000069705. [DOI] [PubMed] [Google Scholar]
- Ogilvie B. M., Love R. J. Co-operation between antibodies and cells in immunity to a nematode parasite. Transplant Rev. 1974;19(0):147–169. doi: 10.1111/j.1600-065x.1974.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Philipp M., Ogilvie B. M. Characterization of surface antigens of Trichinella spiralis infective larvae. Parasite Immunol. 1981 Winter;3(4):339–352. doi: 10.1111/j.1365-3024.1981.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Petit A., Pery P., Luffau G. Purification of an allergen from culture fluids of Nippostrongylus brasiliensis. Mol Immunol. 1980 Nov;17(11):1341–1349. doi: 10.1016/0161-5890(80)90003-6. [DOI] [PubMed] [Google Scholar]
- Philipp M., Parkhouse R. M., Ogilvie B. M. Changing proteins on the surface of a parasitic nematode. Nature. 1980 Oct 9;287(5782):538–540. doi: 10.1038/287538a0. [DOI] [PubMed] [Google Scholar]
- Philipp M., Taylor P. M., Parkhouse R. M., Ogilvie B. M. Immune response to stage-specific surface antigens of the parasitic nematode Trichinella spiralis. J Exp Med. 1981 Jul 1;154(1):210–215. doi: 10.1084/jem.154.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka Z., Mulligan W. Immunological studies on Nippostrongylus brasiliensis infection in the rat: experiments with irradiated larvae. Exp Parasitol. 1965 Aug;17(1):51–56. doi: 10.1016/0014-4894(65)90008-1. [DOI] [PubMed] [Google Scholar]
- SOULSBY E. J. THE NATURE AND ORIGIN OF THE FUNCTIONAL ANTIGENS IN HELMINTH INFECTIONS. Ann N Y Acad Sci. 1963 Dec 30;113:492–509. doi: 10.1111/j.1749-6632.1963.tb40686.x. [DOI] [PubMed] [Google Scholar]
- Siński E., Holmes P. H. Nippostrongylus brasiliensis: systemic and local IgA and IgG immunoglobulin responses in parasitized rats. Exp Parasitol. 1977 Dec;43(2):382–389. doi: 10.1016/0014-4894(77)90044-3. [DOI] [PubMed] [Google Scholar]
- Wilson R. J. Homocytotropic antibody response to the nematode Nippostrongylus brasiliensis in the rat--studies on the worm antigen. J Parasitol. 1967 Aug;53(4):752–762. [PubMed] [Google Scholar]