Abstract
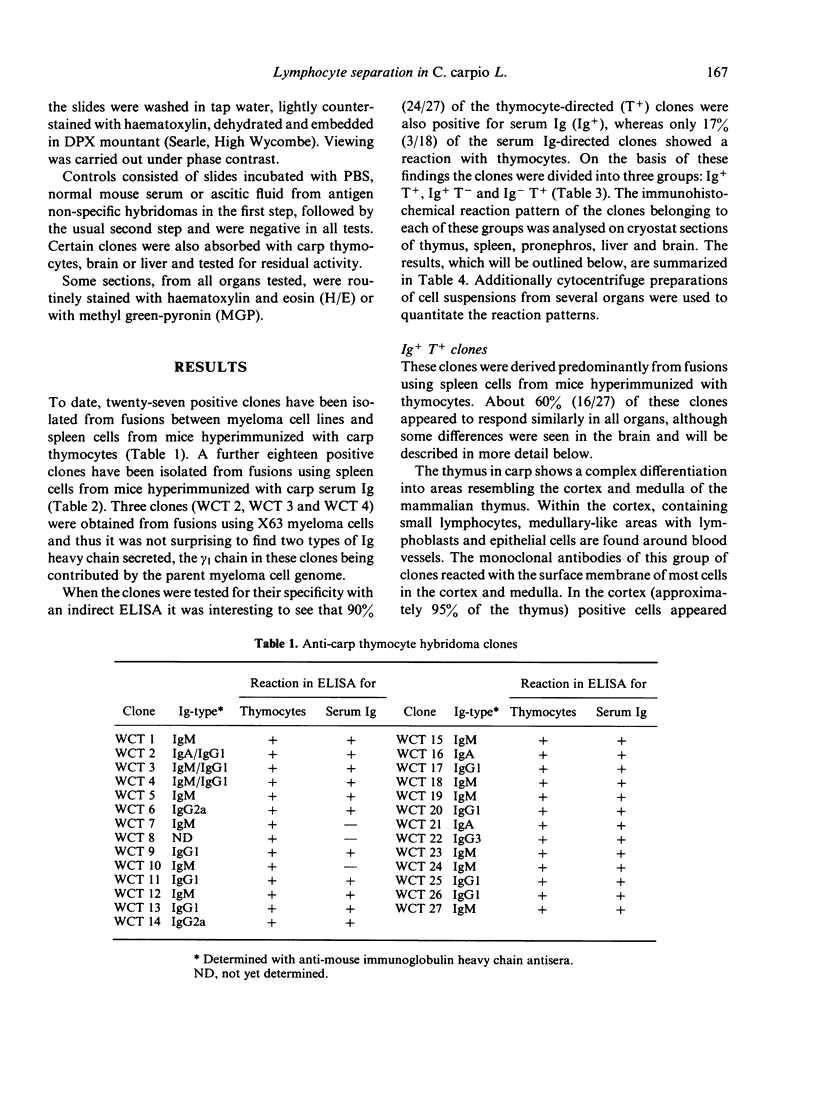
Lymphoid cell populations in various organs of the carp Cyprinus carpio L. were investigated using a series of mouse monoclonal antibodies raised against carp thymocytes and carp serum Ig. Clones have been designated as Ig+T+, Ig+T- or Ig-T+ on the basis of the reactivity with thymocytes and serum Ig in the enzyme-linked immunosorbent assay (ELISA) screening. Their reaction to the lymphoid organs of carp was investigated on cryostat sections and cytocentrifuge slides using immunoperoxidase techniques. IG+T+ clones could be subdivided into those that stained reticular fibres around blood vessels in various organs (R+) and those that did not (R-). The former stained most thymocytes and most peripheral lymphocytes as well as plasma cells whereas the latter did not stain cortical thymocytes and some peripheral lymphocytes. IG+T- clones were negative for thymocytes but positive for plasma cells and a certain population of peripheral lymphocytes. Ig-T+ clones reacted similarly to Ig+T+R- clones. It is concluded that fish lymphoid cell populations can be distinguished based upon differences in cell surface and/or cytoplasmic determinants. The monoclonal antibodies described can be used for further structural analysis of the determinants and for functional separation of T- and B-like cells in the 'lower' vertebrates.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avtalion R. R., Weiss E., Moalem T., Milgrom L. Proceedings: Evidence for cooperation of T and B cells in fish. Isr J Med Sci. 1975 Dec;11(12):1385–1385. [PubMed] [Google Scholar]
- Barclay A. N. Different reticular elements in rat lymphoid tissue identified by localization of Ia, Thy-1 and MRC OX 2 antigens. Immunology. 1981 Dec;44(4):727–736. [PMC free article] [PubMed] [Google Scholar]
- Binz H., Wigzell H. Antigen-binding, idiotypic T-lymphocyte receptors. Contemp Top Immunobiol. 1977;7:113–177. doi: 10.1007/978-1-4684-3054-7_4. [DOI] [PubMed] [Google Scholar]
- Bodger M. P., Francis G. E., Delia D., Granger S. M., Janossy G. A monoclonal antibody specific for immature human hemopoietic cells and T lineage cells. J Immunol. 1981 Dec;127(6):2269–2274. [PubMed] [Google Scholar]
- Brockes J. P., Fields K. L., Raff M. C. A surface antigenic marker for rat Schwann cells. Nature. 1977 Mar 24;266(5600):364–366. doi: 10.1038/266364a0. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem L. W., McLean W. E., Shankey V. T., Cuchens M. A. Phylogeny of lymphocyte heterogeneity. I. Membrane immunoglobulins of teleost lymphocytes. Dev Comp Immunol. 1977 Apr;1(2):105–118. doi: 10.1016/s0145-305x(77)80004-9. [DOI] [PubMed] [Google Scholar]
- Cuchens M. A., Clem L. W. Phylogeny of lymphocyte heterogeneity. II. Differential effects of temperature on fish T-like and B-like cells. Cell Immunol. 1977 Dec;34(2):219–230. doi: 10.1016/0008-8749(77)90245-3. [DOI] [PubMed] [Google Scholar]
- DeLuca D., Warr G. W., Marchalonis J. J. Phylogenetic origins of immune recognition: lymphocyte surface immunoglobulins and antigen binding in the genus Carassius (Teleostii). Eur J Immunol. 1978 Jul;8(7):525–530. doi: 10.1002/eji.1830080713. [DOI] [PubMed] [Google Scholar]
- Eichmann K., Ben-Neriah Y., Hetzelberger D., Polke C., Givol D., Lonai P. Correlated expression of VH framework and VH idiotypic determinants on T helper cells and on functionally undefined T cells binding group A streptococcal carbohydrate. Eur J Immunol. 1980 Feb;10(2):105–112. doi: 10.1002/eji.1830100207. [DOI] [PubMed] [Google Scholar]
- Ellis A. E., Parkhouse R. M. Surface immunoglobulins on the lymphocytes of the skate Raja naevus. Eur J Immunol. 1975 Oct;5(10):726–728. doi: 10.1002/eji.1830051014. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Richter R. F., Ambrosius H. Immunoglobulin determinants on the surface of lymphoid cells of carps. Eur J Immunol. 1975 Jan;5(1):76–78. doi: 10.1002/eji.1830050118. [DOI] [PubMed] [Google Scholar]
- Etlinger H. M., Hodgins H. O., Chiller J. M. Evolution of the lymphoid system. I. Evidence for lymphocyte heterogeneity in rainbow trout revealed by the organ distribution of mitogenic responses. J Immunol. 1976 Jun;116(6):1547–1553. [PubMed] [Google Scholar]
- Goldschneider I., Gordon L. K., Morris R. J. Demonstration of Thy-1 antigen on pluripotent hemopoietic stem cells in the rat. J Exp Med. 1978 Nov 1;148(5):1351–1366. doi: 10.1084/jem.148.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb C. J., Clem L. W. Fish lymphocytes differ in the expression of surface immunoglobulin. Dev Comp Immunol. 1982 Summer;6(3):473–479. doi: 10.1016/s0145-305x(82)80033-5. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Pillemer E., Weissman I. L. A monoclonal antibody that detects a V kappa-TEPC15 idiotypic determinant cross-reactive with a Thy-1 determinant. J Exp Med. 1981 May 1;153(5):1068–1079. doi: 10.1084/jem.153.5.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajewsky K., Eichmann K. Antigen receptors of T helper cells. Contemp Top Immunobiol. 1977;7:69–112. doi: 10.1007/978-1-4684-3054-7_3. [DOI] [PubMed] [Google Scholar]
- Ritter M. A., Gordon L. K., Goldschneider I. Distribution of identity of Thy-1-bearing cells during ontogeny in rat hemopoietic and lymphoid tissues. J Immunol. 1978 Dec;121(6):2463–2471. [PubMed] [Google Scholar]
- Stolen J. S., Mäkelä O. Carrier preimmunisation in the anti-hapten response of a marine fish. Nature. 1975 Apr 24;254(5502):718–719. doi: 10.1038/254718a0. [DOI] [PubMed] [Google Scholar]
- Warr G. W., DeLuca D., Marchalonis J. J. Phylogenetic origins of immune recognition: lymphocyte surface immunoglobulins in the goldfish, Carassius auratus. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2476–2480. doi: 10.1073/pnas.73.7.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaga K. M., Kubo R. T., Etlinger H. M. Studies on the question of conventional immunoglobulin on thymocytes from primitive vertebrates. II. Delineation between Ig-specific and cross-reactive membrane components. J Immunol. 1978 Jun;120(6):2074–2079. [PubMed] [Google Scholar]
- van Ewijk W., van Soest P. L., van den Engh G. J. Fluorescence analysis and anatomic distribution of mouse T lymphocyte subsets defined by monoclonal antibodies to the antigens Thy-1, Lyt-1, Lyt-2, and T-200. J Immunol. 1981 Dec;127(6):2594–2604. [PubMed] [Google Scholar]