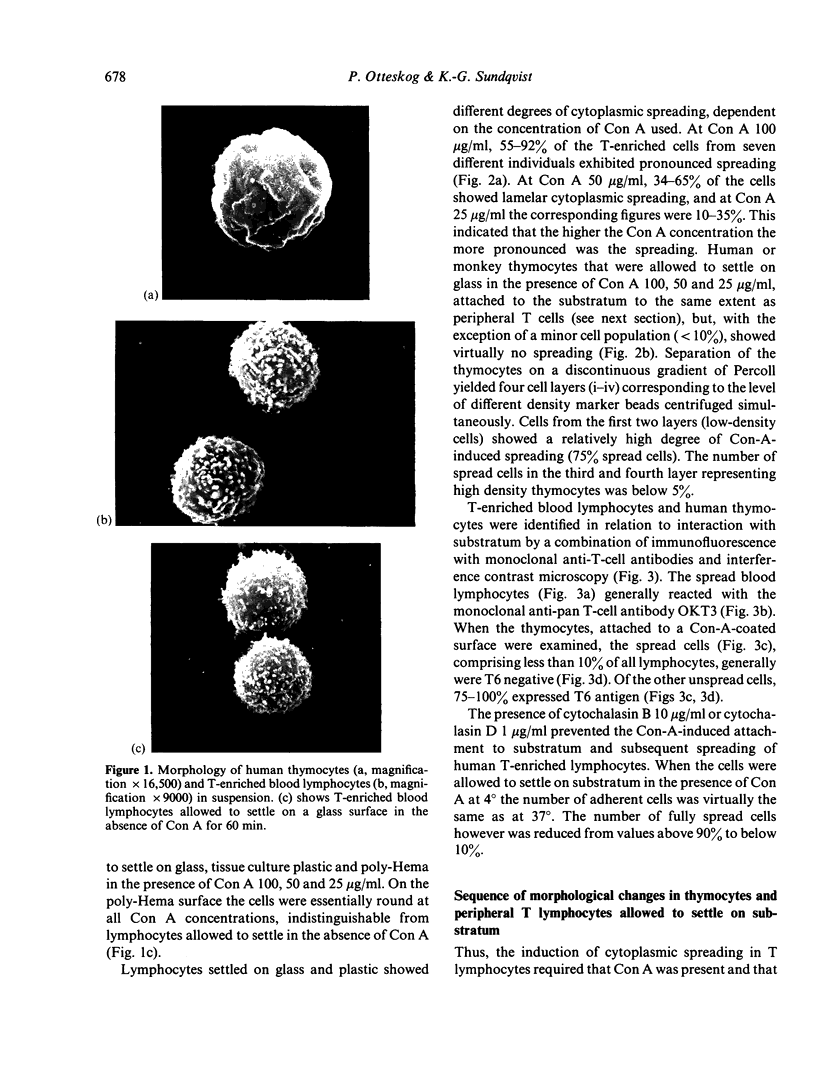
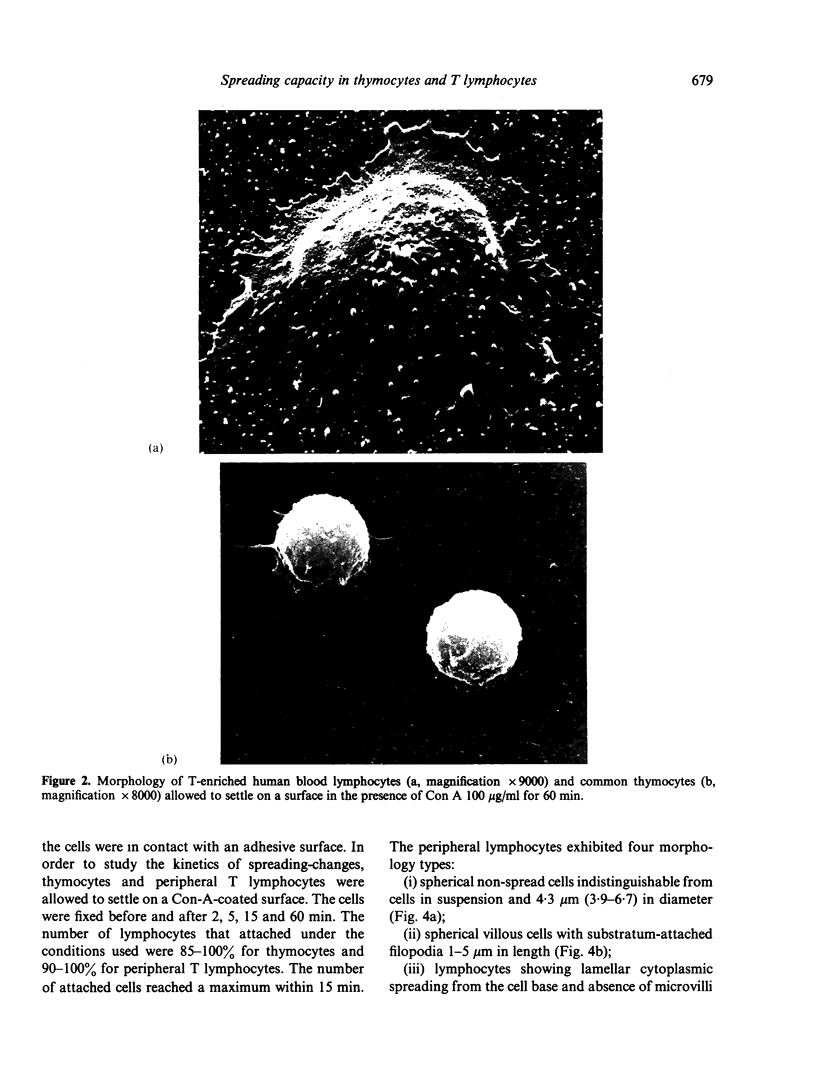
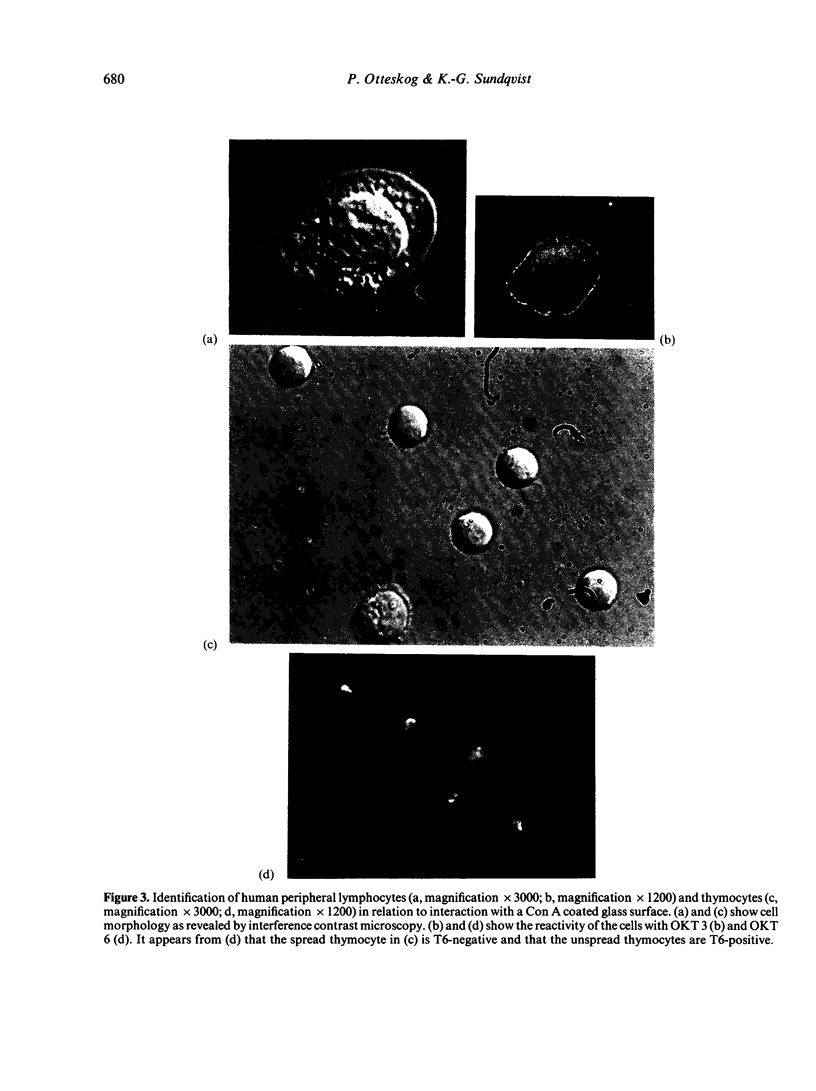
Abstract
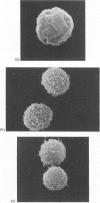
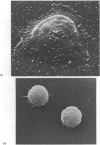
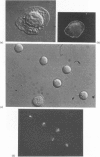
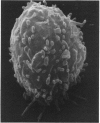
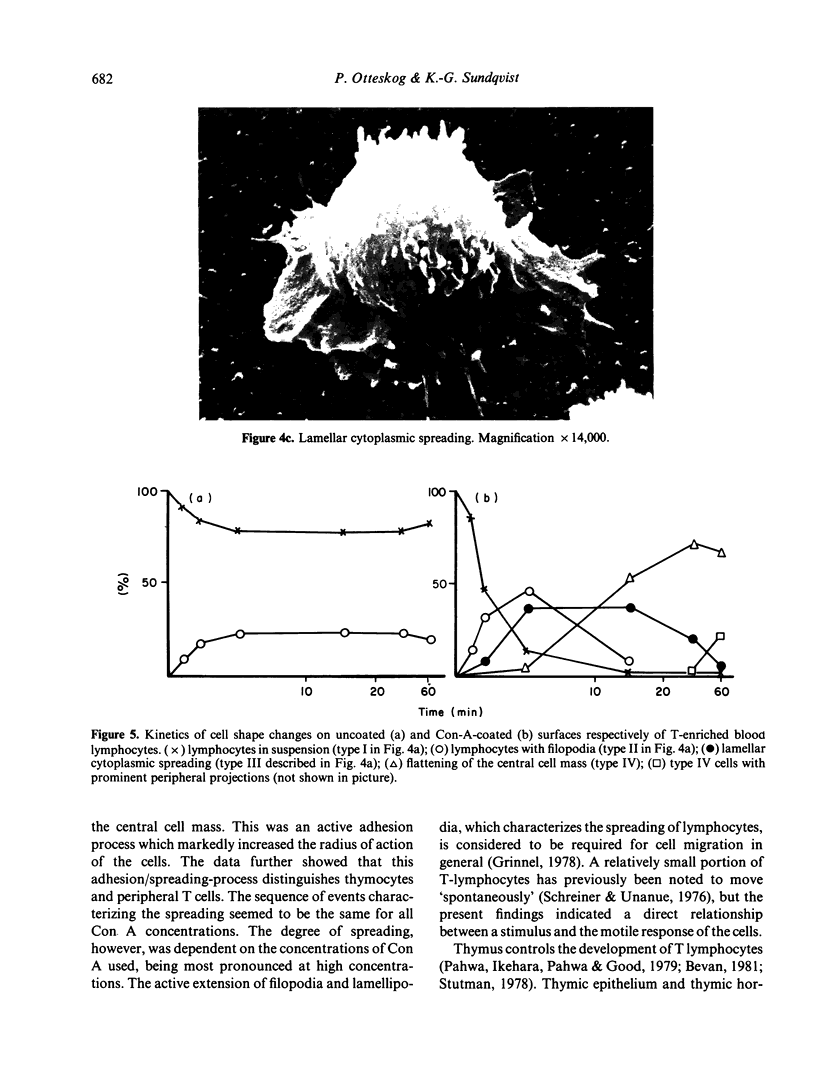
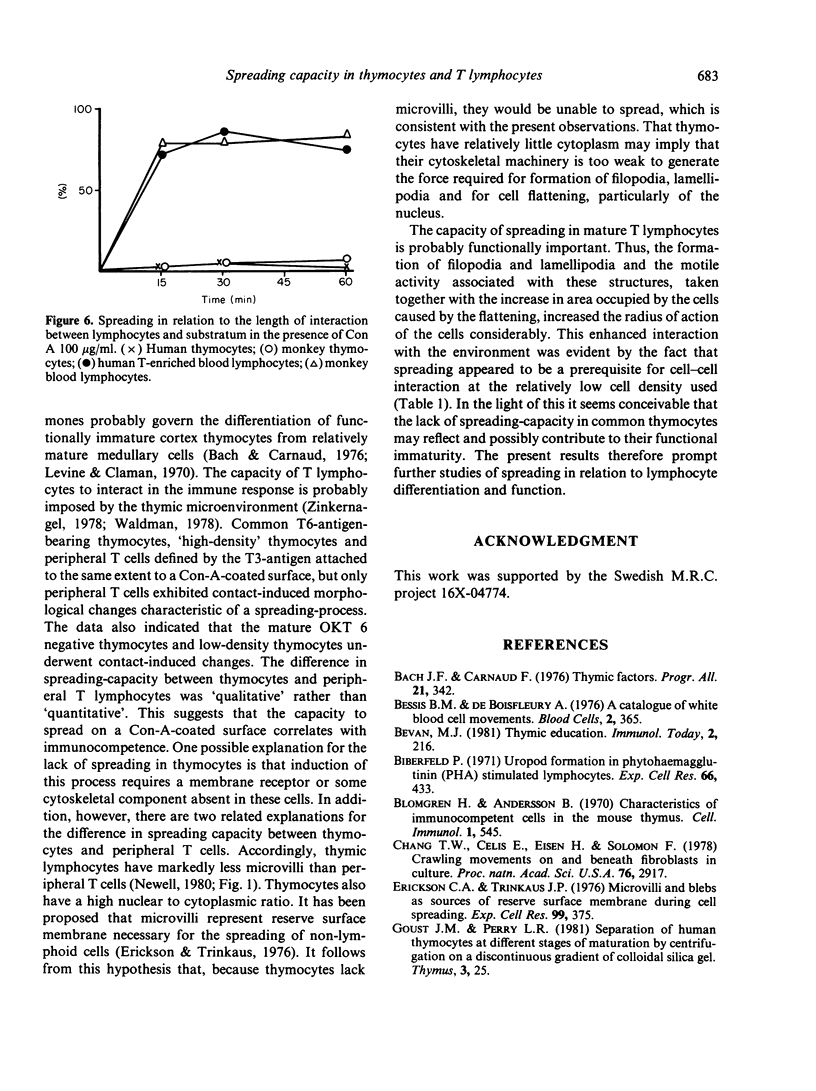
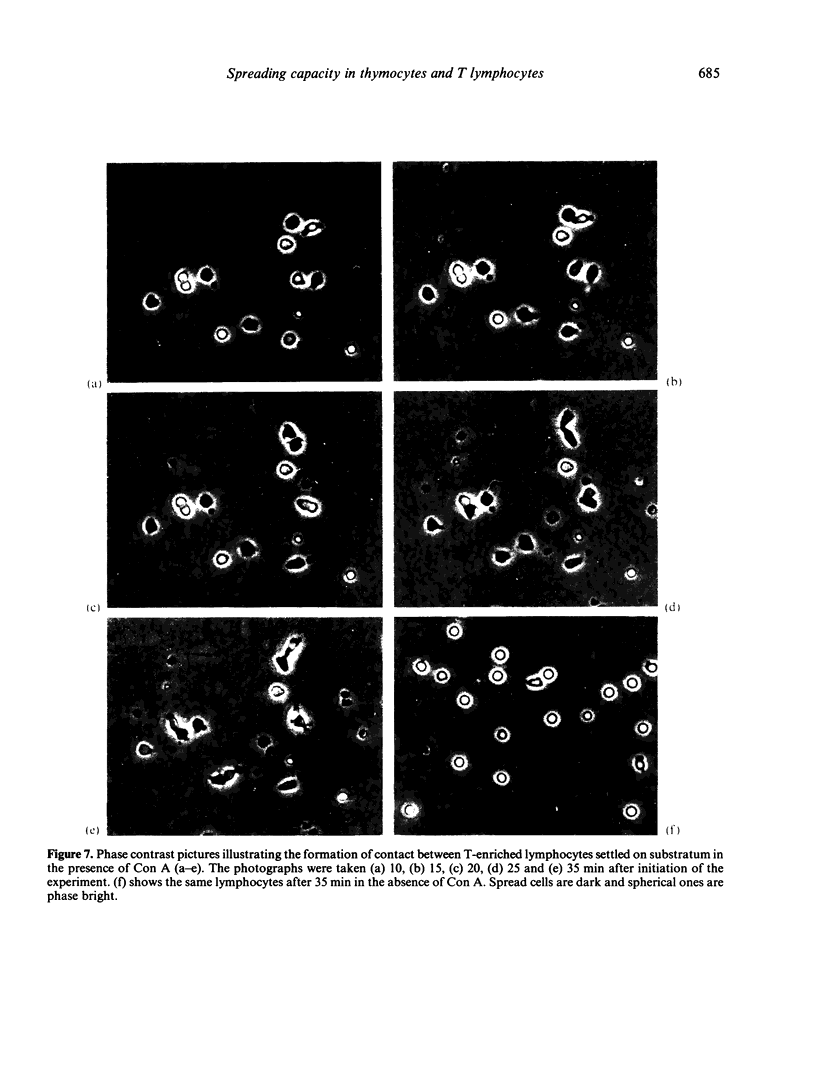
Contact of T-enriched human blood lymphocytes with an adhesive surface in the presence of Concanavalin A (Con A) almost immediately induced a sequence of motile changes in virtually all cells. The initial event in this spreading process was the formation of filopodia distinct from the microvilli of lymphocytes in suspension. The filopodia were accompanied by lamellipodia, ruffles and flattening of the nucleus. Contact with a nonadhesive substratum in the presence of Con A did not trigger this sequence of changes. Cytochalasin B and D or low temperature inhibited the contact-induced changes. With the exception of a small number of cells (5-15%), T-enriched lymphocytes that were allowed to settle in the absence of Con A showed a radius of action (area occupied by the cells/translational movement per hr) of 39 micrometers 2/ less than 1 micrometer. The small 'motile' population showed a radius of action of 74 micrometers 2/8 micrometers. The Con-A-mediated spreading-process yielded a radius of action of the lymphocytes of 117 micrometers 2/6 micrometers. This augmented radius of action markedly facilitated cell-cell interaction in a high frequency of the cells and appeared to be a prerequisite for such interactions at 'low' cell density. Thymocytes reactive with OKT 6 antibodies or belonging to the 'high-density' fraction of cells attached to a Con-A-coated surface to the same extent as peripheral OKT 3 positive lymphocytes, but did not exhibit the morphological changes characteristic of a spreading-process. In contrast, OKT 6 negative thymocytes or thymocytes with a relatively low density showed spreading indistinguishable from that of OKT 3 positive peripheral lymphocytes. These results characterize the spreading-process in human T lymphocytes and demonstrate its functional importance for interactions with the environment. Spreading-capacity appears to reflect the stage of maturation of T cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach J. F., Carnaud C. Thymic factors. Prog Allergy. 1976;21:342–408. doi: 10.1159/000399402. [DOI] [PubMed] [Google Scholar]
- Biberfeld P. Uropod formation in phytohaemagglutinin (PHA) stimulated lymphocytes. Exp Cell Res. 1971 Jun;66(2):433–445. doi: 10.1016/0014-4827(71)90698-7. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Andersson B. Characteristics of the immunocompetent cells in the mouse thymus: cell population changes during cortisone-induced atrophy and subsequent regeneration. Cell Immunol. 1970 Nov;1(5):545–560. doi: 10.1016/0008-8749(70)90041-9. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Celis E., Eisen H. N., Solomon F. Crawling movements of lymphocytes on and beneath fibroblasts in culture. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2917–2921. doi: 10.1073/pnas.76.6.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson C. A., Trinkaus J. P. Microvilli and blebs as sources of reserve surface membrane during cell spreading. Exp Cell Res. 1976 May;99(2):375–384. doi: 10.1016/0014-4827(76)90595-4. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Cellular adhesiveness and extracellular substrata. Int Rev Cytol. 1978;53:65–144. doi: 10.1016/s0074-7696(08)62241-x. [DOI] [PubMed] [Google Scholar]
- Levine M. A., Claman H. N. Bone marrow and spleen: dissociation of immunologic properties by cortisone. Science. 1970 Mar 13;167(3924):1515–1517. doi: 10.1126/science.167.3924.1515. [DOI] [PubMed] [Google Scholar]
- O'Neill G. J., Parrott D. M. Locomotion of human lymphoid cells. I. Effect of culture and con A on T and non-T lymphocytes. Cell Immunol. 1977 Oct;33(2):257–267. doi: 10.1016/0008-8749(77)90156-3. [DOI] [PubMed] [Google Scholar]
- Papiernik M., Laroche L., Bach J. F. Thymocyte subpopulations in young and adult mice. I. Separation by density gradient and steroid treatment. Eur J Immunol. 1977 Nov;7(11):796–799. doi: 10.1002/eji.1830071110. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Membrane and cytoplasmic changes in B lymphocytes induced by ligand-surface immunoglobulin interaction. Adv Immunol. 1976;24:37–165. doi: 10.1016/s0065-2776(08)60329-6. [DOI] [PubMed] [Google Scholar]
- Stutman O. Intrathymic and extrathymic T cell maturation. Immunol Rev. 1978;42:138–184. doi: 10.1111/j.1600-065x.1978.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Sundqvist K. G., Otteskog P., Wanger L., Thorstensson R., Utter G. Morphology and microfilament organization in human blood lymphocytes. Effects of substratum and mitogen exposure. Exp Cell Res. 1980 Dec;130(2):327–337. doi: 10.1016/0014-4827(80)90009-9. [DOI] [PubMed] [Google Scholar]
- Sundqvist K. G., Wanger L. Anchorage and lymphocyte function II. Contact with non-cellular surfaces, cell density and T-cell activation. Immunology. 1981 Jul;43(3):573–580. [PMC free article] [PubMed] [Google Scholar]
- Sundqvist K. G., Wanger L. Anchorage and lymphocyte function. Contact-induced augmentation of T-cell activation. Immunology. 1980 Dec;41(4):883–890. [PMC free article] [PubMed] [Google Scholar]
- Waldmann H. The influence of the major histocompatibility complex on the function of T-helper cells in antibody formation. Immunol Rev. 1978;42:202–223. doi: 10.1111/j.1600-065x.1978.tb00263.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. C., Parrott D. M., Russell R. J., Sless F. Antigen-induced locomotor responses in lymphocytes. J Exp Med. 1977 May 1;145(5):1158–1168. doi: 10.1084/jem.145.5.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]