Abstract
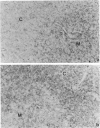
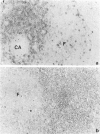
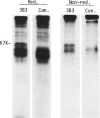
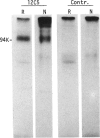
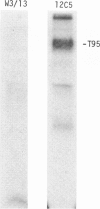
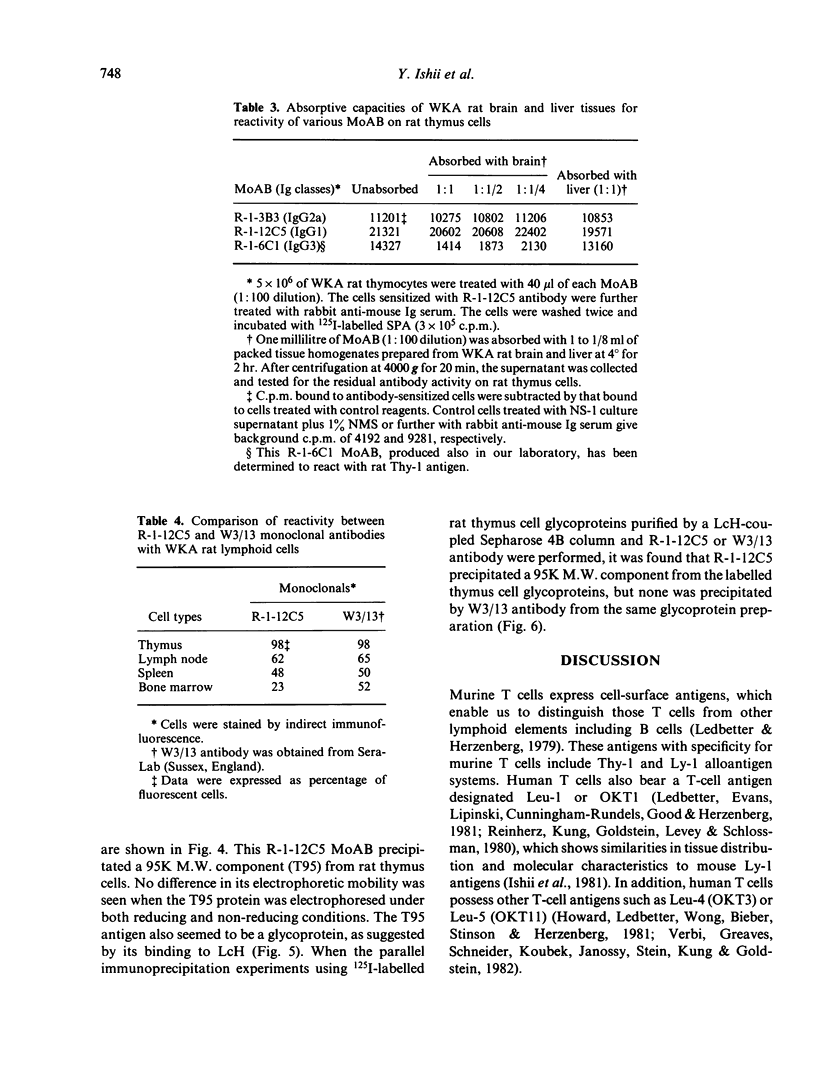
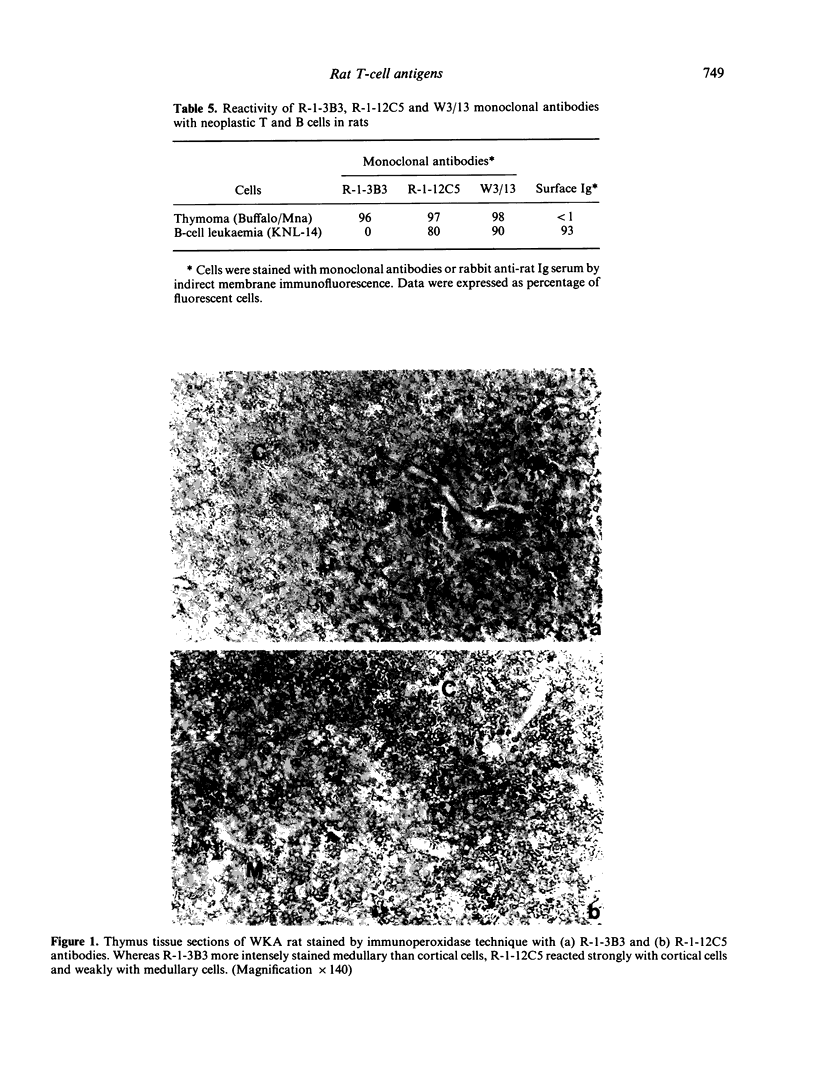
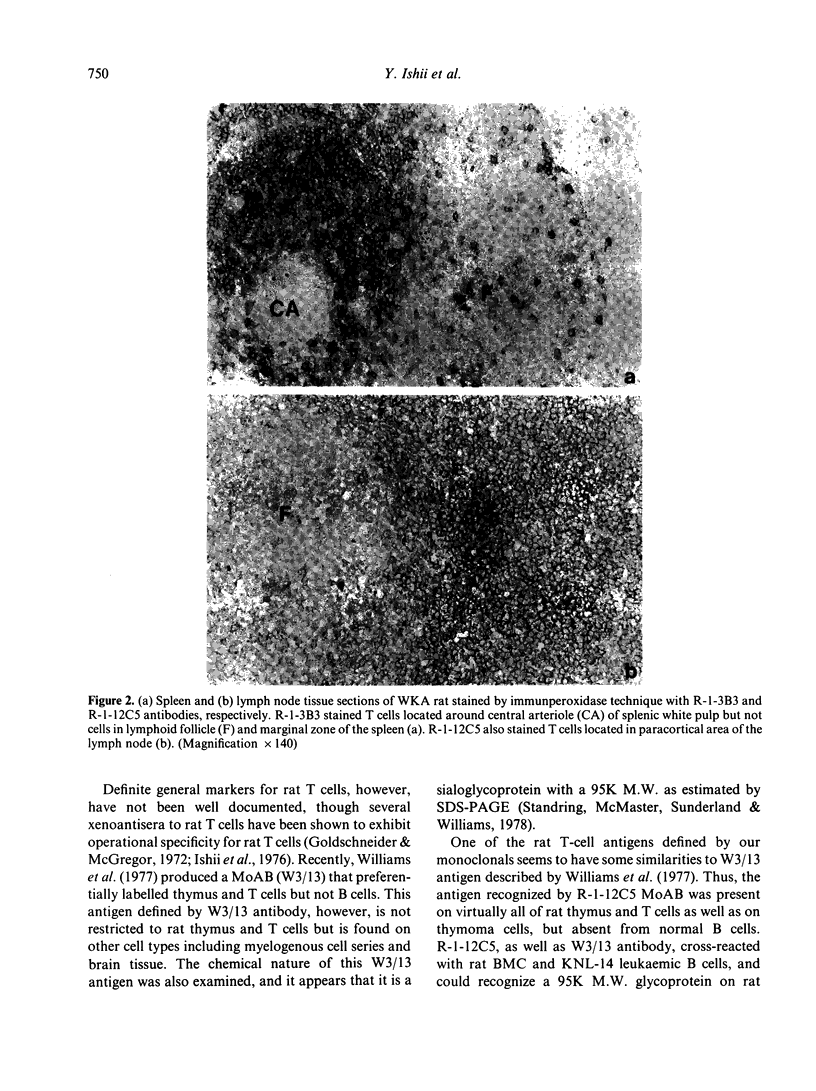
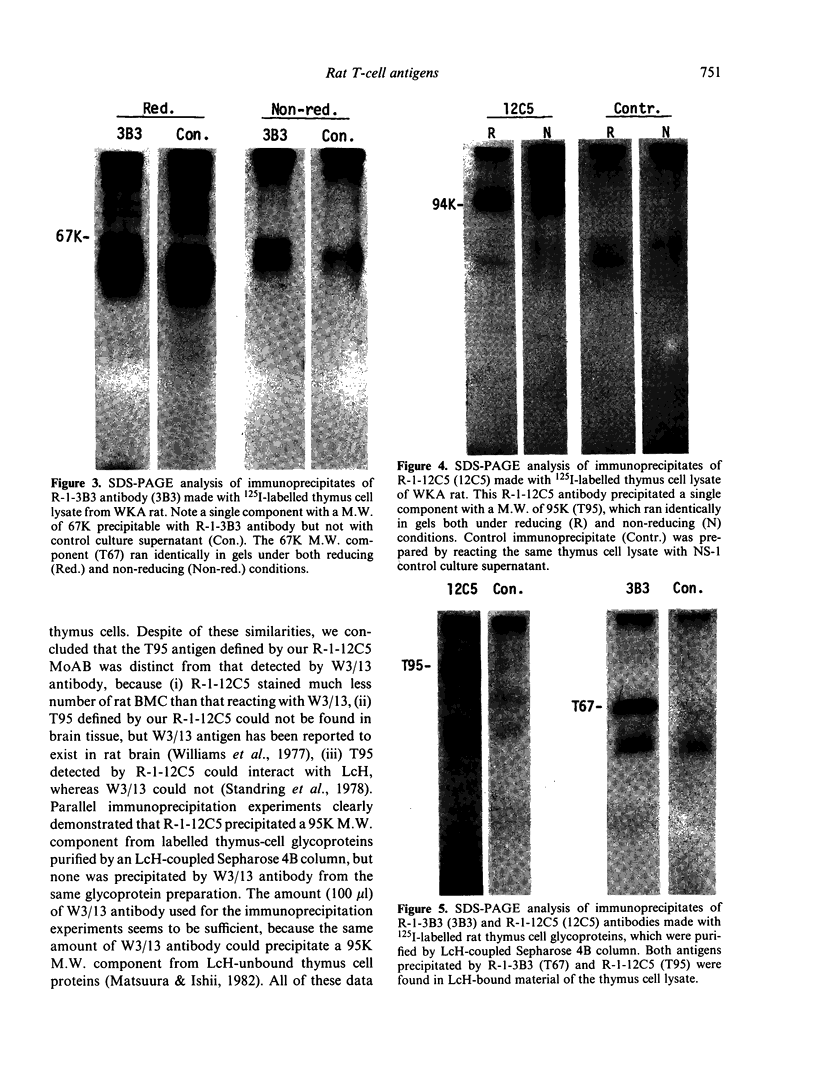
Rat thymus and T-cell antigens were defined by using two distinct monoclonal antibodies (R-1-3B3 and R-1-12C5). R-1-3B3 antibody, when tested for its reactivity with rat lymphoid cells by immunofluorescence, labelled virtually all thymus and T cells but not B cells and bone marrow cells. The antigen defined by this R-1-3B3 antibody occurred more abundantly on medullary thymocytes and peripheral T cells than on cortical thymocytes. Immunochemical data showed that R-1-3B3 antibody recognized a single glycoprotein with a molecular weight of 67,000, which were able to interact with Lens culinaris haemagglutinin. R-1-12C5 antibody, on the other hand, reacted with all of thymus and T cells as well as with a subpopulation (approximately 20%) of bone marrow cells. In contrast to the antigen defined by R-1-3B3, that detected by R-1-12C5 antibody existed largely on cortical thymocytes and to a much lesser extent on medullary thymocytes and peripheral T cells. R-1-12C5 antibody detected a single glycoprotein with a 95,000 molecular weight, which could also interact with Lens culinaris haemagglutinin. Based on these data described above and since both antigens defined by R-1-3B3 and R-1-12C5 antibodies were absent from rat brain tissue, we concluded that they were distinct from brain-associated thymic antigens in rats including Thy-1 and W3/13 antigen systems.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ades E. W., Zwerner R. K., Acton R. T., Balch C. M. Isolation and partial characterization of the human homologue of Thy-1. J Exp Med. 1980 Feb 1;151(2):400–406. doi: 10.1084/jem.151.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay A. N. The localization of populations of lymphocytes defined by monoclonal antibodies in rat lymphoid tissues. Immunology. 1981 Apr;42(4):593–600. [PMC free article] [PubMed] [Google Scholar]
- Boyse E. A., Miyazawa M., Aoki T., Old L. J. Ly-A and Ly-B: two systems of lymphocyte isoantigens in the mouse. Proc R Soc Lond B Biol Sci. 1968 Jun 11;170(1019):175–193. doi: 10.1098/rspb.1968.0032. [DOI] [PubMed] [Google Scholar]
- Crawford J. M., Goldschneider I. THY1 antigen and B lymphocyte differentiation in the rat. J Immunol. 1980 Feb;124(2):969–976. [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. J Immunol. 1976 Jul;117(1):136–142. [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification and unusual tissue distribution of the canine and human homologues of Thy-1 (theta). J Exp Med. 1979 Mar 1;149(3):576–591. doi: 10.1084/jem.149.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durda P. J., Shapiro C., Gottlieb P. D. Partial molecular characterization of the Ly-1 alloantigen on mouse thymocytes. J Immunol. 1978 Jan;120(1):53–57. [PubMed] [Google Scholar]
- Goldschneider I., McGregor D. D. Anatomical distribution of T and B lymphocytes in the rat. Development of lymphocyte-specific antisera. J Exp Med. 1973 Dec 1;138(6):1443–1465. doi: 10.1084/jem.138.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard F. D., Ledbetter J. A., Wong J., Bieber C. P., Stinson E. B., Herzenberg L. A. A human T lymphocyte differentiation marker defined by monoclonal antibodies that block E-rosette formation. J Immunol. 1981 Jun;126(6):2117–2122. [PubMed] [Google Scholar]
- Hunt S. V. The presence of Thy-1 on the surface of rat lymphoid stem cells and colony-forming units. Eur J Immunol. 1979 Nov;9(11):853–859. doi: 10.1002/eji.1830091105. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Fujimoto J., Kon S., Ogasawara M., Koshiba H., Matsuura A., Kikuchi K. Immunochemical characterization of human TL-like (T48) and Ly 1-like (T72) glycoproteins using two-dimensional polyacrylamide gel electrophoresis. Clin Exp Immunol. 1981 Dec;46(3):530–540. [PMC free article] [PubMed] [Google Scholar]
- Ishii Y., Fujimoto J., Koshiba H., Kikuchi K. Isolation and partial characterization of a 72,000-dalton glycoprotein (Tgp72) on human thymus and T cells: possible relationship to mouse Ly-1 antigens. J Immunol. 1981 Jun;126(6):2171–2176. [PubMed] [Google Scholar]
- Ishii Y., Koshiba H., Yamaoka H., Kikuchi K. Rat T lymphocyte-specific antigens and their cross-reactivity with mouse T cells. J Immunol. 1976 Aug;117(2):497–503. [PubMed] [Google Scholar]
- Ishii Y., Ueno H., Kikuchi K. Ultrastructure of lymphoid cells with surface-bound immunoglobulin in rats. J Reticuloendothel Soc. 1974 Feb;15(2):155–162. [PubMed] [Google Scholar]
- Itakura K., Hutton J. J., Boyse E. A., Old L. J. Genetic linkage relationships of loci specifying differentiation alloantigens in the mouse. Transplantation. 1972 Mar;13(3):239–243. doi: 10.1097/00007890-197203000-00007. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kumararatne D. S., Bazin H., MacLennan I. C. Marginal zones: the major B cell compartment of rat spleens. Eur J Immunol. 1981 Nov;11(11):858–864. doi: 10.1002/eji.1830111103. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke H., Hammerling G. J., Hohmann C., Rajewsky K. Hybrid cell lines secreting monoclonal antibody specific for major histocompatibility antigens of the mouse. Nature. 1978 Jan 19;271(5642):249–251. doi: 10.1038/271249a0. [DOI] [PubMed] [Google Scholar]
- McCabe R. P., Quaranta V., Frugis L., Ferrone S., Reisfeld R. A. A radioimmunometric antibody-binding assay for evaluation of xenoantisera to melanoma-associated antigens. J Natl Cancer Inst. 1979 Mar;62(3):455–463. doi: 10.1093/jnci/62.3.455. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring R., McMaster W. R., Sunderland C. A., Williams A. F. The predominant heavily glycosylated glycoproteins at the surface of rat lymphoid cells are differentiation antigens. Eur J Immunol. 1978 Dec;8(12):832–839. doi: 10.1002/eji.1830081203. [DOI] [PubMed] [Google Scholar]
- Uede T., Ishii Y., Matsuura A., Shimogawara I., Kikuchi K. Immunohistochemical study of lymphocytes in rat pineal gland: selective accumulation of T lymphocytes. Anat Rec. 1981 Feb;199(2):239–247. doi: 10.1002/ar.1091990208. [DOI] [PubMed] [Google Scholar]
- Verbi W., Greaves M. F., Schneider C., Koubek K., Janossy G., Stein H., Kung P., Goldstein G. Monoclonal antibodies OKT 11 and OKT 11A have pan-T reactivity and block sheep erythrocyte "receptors". Eur J Immunol. 1982 Jan;12(1):81–86. doi: 10.1002/eji.1830120115. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Williams A. F. Many cells in rat bone marrow have cell-surface Thy-1 antigen. Eur J Immunol. 1976 Jul;6(7):526–528. doi: 10.1002/eji.1830060716. [DOI] [PubMed] [Google Scholar]
- Yamanaka N., Ishii Y., Koshiba H., Kikuchi K. Immunohistological detection of B lymphocytes in the rat lymphoid organs by using anti-rat B lymphocyte serum. Tohoku J Exp Med. 1981 Oct;135(2):187–196. doi: 10.1620/tjem.135.187. [DOI] [PubMed] [Google Scholar]